Abstract
Background: Hemodialysis (HD) and plasmapheresis (PE) are usually performed independently on patients who require renal replacement therapy. We analyzed our experience using a technique that performs both modalities simultaneously. Methods: Thirty-six patients who were treated with 287 tandem PE and HD (TPH) sessions (mean 7.97 ± 5.6 per patient) were included. PE was connected 30 min after HD started. The mean HD blood flow was 313.7 ± 44 mL/min, the mean PE blood flow was 141 ± 25 mL/min, and the duration of TPH was no longer than 240 min. The heparin dose was similar to that used for a standard HD procedure. Results: In 287 TPH sessions performed, 10.45% experienced minor complications. There were significant changes in mean blood pressure after connection of the PE system. However, these differences were not clinically relevant since patients remained asymptomatic and they did not require saline infusion. At the end of treatment, 38.9% of patients were no longer dependent on dialysis. Conclusions: Our results suggest that TPH is a safe and effective treatment that decreases exposure to an extracorporeal circuit, reducing the risks that are associated with anticoagulation agents and improving the comfortability of the patient.
INTRODUCTION
Plasmapheresis (PE) is an effective technique for the removal of antibodies and immunocomplexes.Citation1,2 There are several diseases, such as Goodpasture’s syndrome,Citation1,3,4 rapidly progressive glomerulonephritis (RPGN),Citation1,2,5–8 thrombotic microangiopathy (TMA)Citation9 (including hemolytic uremic syndromeCitation10 and thrombotic thrombocytopenic purpura), and acute humoral rejection (AHR),Citation11–13 for which PE is indicated.
Many patients with these immune-mediated diseases have renal failure requiring renal replacement therapy with hemodialysis (HD) in addition to plasma exchange. In these patients, HD and PE are usually performed independently,Citation1,2 prolonging the exposure to an extracorporeal circuit and, consequently, increasing the anticoagulation dose.Citation14 Moreover, when the techniques are performed separately, there is an increase in personnel costs and the patient’s comfort is negatively impacted.Citation15–18
Several authors have described small case series of tandem PE and HD (TPH).Citation15,16 Our group has designed a clinical protocol for TPH. The aim of this study was to analyze our experience with this procedure in the past 12 years.
PATIENTS AND METHODS
We performed an observational study of 36 patients who were treated with a total of 287 TPH sessions between January 1998 and February 2010 in our center. Demographic variables, indications for the technique, renal survival, and all adverse events were recorded.
The mean age of the patients was 54.94 ± 14.9 years; 58.3% (n = 21) were male. A total of 287 sessions were performed, ranging from 3 to 31 per patient, with a mean of 7.97 ± 5.6. Indications for PE were TMA (hemolytic uremic syndrome or thrombotic thrombocytopenic purpura) (3 cases, 8.3%), RPGN (21 cases, 58.3%), AHR (6 cases, 16.7%), antiglomerular basement membrane disease (6 cases, 16.7%). Multiple myeloma patients were not included in this study.
Technical Features
Vascular access and plasma exchange solution
The vascular access for all cases was a temporary double-lumen catheter, whose length depended on the location (right/left femoral, 20 cm; left jugular, 20 cm; and right jugular, 15 cm). All patients began with a femoral catheter, which was replaced by a jugular catheter if the procedure lasted for more than 7 days.
Plasma exchange was performed using fresh frozen plasma (FFP) for TMA or purified lyophilized plasma (PLP) for the rest of the patients.
TPH setup
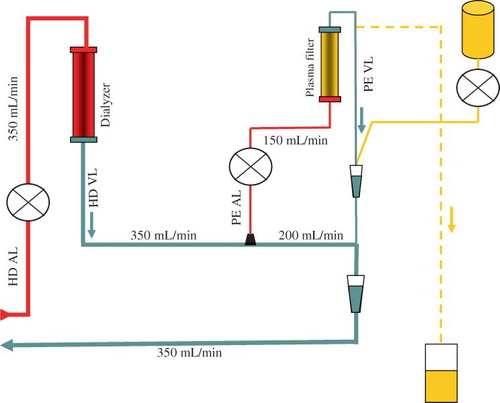
A conventional HD system was used. The dialyzer was a high-flux membrane (polysulfone and helixone) in 10 patients and low-flux membrane (polyamide) in 26 patients. The blood flow prescribed was 350–400 mL/min and the dialysate flow was 500 mL/min. TPH procedure required an additional venous blood line with a Luer-lock® connection, placed between the output of the dialyzer and the venous pressure chamber that provided access to the blood circuit for the PE (). The returning blood line from the PE circuit was connected to the venous pressure chamber. For PE, the blood flow was 150 mL/min. The plasma filter was a polysulfone with a surface of 0.6 m2 and a pore size of 0.6 μm.
Anticoagulation
Anticoagulation of the extracorporeal circuit was performed with an initial bolus of 1% sodium heparin (mean 21 ± 16 mg per session). No additional heparin was used when the PE system was started.
Allergy prophylaxis
Premedication included dexchlorpheniramine, methylprednisolone, and oral calcium supplements.
Clinical Features
Blood pressure, heart rate, and temperature were monitored and documented every hour. The hemodynamic tolerance was analyzed; mean blood pressure (MBP) was calculated at four points: 30 min after HD was initiated (prePE), immediately after connection of PE (PE0), 1 h after PE was begun (PE1), and at the end of PE (postPE), when the PE system was closed.
Statistical Analysis
A descriptive analysis of the results was performed by calculating mean values ± standard deviations. The χ2 test was used to analyze the categorical variables, and analysis of variance (ANOVA) was used to analyze hemodynamic tolerance. A p < 0.05 was considered statistically significant. The data were analyzed by SPSS v 15.0 software package for Windows (SPSS, Chicago, IL, USA).
RESULTS
Mean blood flow during HD was 313.7 ± 44 mL/min. In the PE circuit, the blood flow was 141 ± 25 mL/min. The plasma exchange flow was 20–40 mL/min (median 30 mL/min), with a mean volume exchange of 45 mL/kg (2500–3500; median 3000 mL). The transmembrane pressure of PE was always less than 100 mmHg. The total duration of TPH was 180–240 min. In all cases, PE was connected 30 min after the initiation of the HD circuit.
Adverse Events
Three of 36 patients died during their inpatient stay—one had Goodpasture’s disease with active pulmonary bleeding and brain hemorrhage; another had a microscopic polyangiitis with active pulmonary hemorrhage; and the third had a cryoglobulinemic vasculitis. No deaths occurred during TPH.
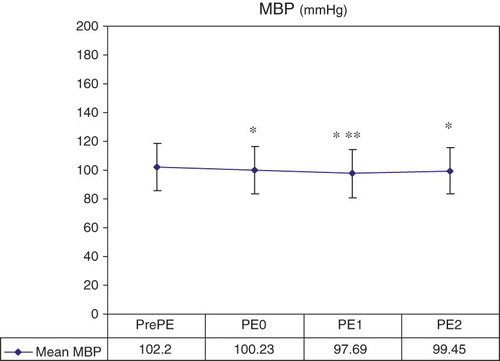
We analyzed 287 TPH sessions; 10.45% experienced minor complications as detailed in , 4.52% of which were possibly attributed to the simultaneous procedure (symptomatic hypotension 3.83% and extracorporeal circuit clotting 0.69%). The MBP decreased significantly when PE was connected [PE0 = 100.23 ± 16.9 mmHg (p = 0.001) vs. prePE = 102.2 ± 16.65 mmHg] and remained significantly decreased along the PE procedure [vs. PE1 = 97.69 ± 16.7 mmHg (p < 0.001) and vs. postPE = 99.45 ± 16.19 mmHg (p < 0.001)]. There were also significant differences between PE0 and PE1 (p = 0.001; ). However, these differences were not clinically relevant since patients remained asymptomatic and they did not require saline infusion or any other vasopressor agent.
Table 1. Adverse events registered during 287 tandem PE and HD (TPH) sessions and with FFP and PLP separately.
Figure 2. Hemodynamic tolerance.
Notes: MBP, mean blood pressure; PE, plasmapheresis; HD, hemodialysis; PrePE, after HD was initiated and previously PE was connected; PE0, at the moment of PE connection; PE1, 1 h before PE started; PE2, at the end of PE, with the circuit ended.
*PrePE versus PE0 (p = 0.001), versus PE1 (p < 0.001), and versus PE2 (p < 0.001).
**PE0 versus PE1 (p = 0.001).
We also analyzed the adverse events with both plasma replacement modalities (FFP and PLP) separately. A total of 79 sessions were performed with FFP, and adverse events were observed in 12 of them (15.18%); 208 sessions were performed with PLP, and adverse events occurred in 18 (8.65%). There were no significant differences between the modalities. Paresthesias occurred in 2 (0.69%) of 287 sessions. Notably, two episodes of paresthesias occurred only in patients whose plasma exchange was performed using FFP (p = 0.033). All reported adverse reactions are shown in .
Renal Survival
Regardless of underlying disease, 38.9% (n = 14) of patients were no longer dependent on dialysis after treatment, and 61.1% (n = 22) still required renal replacement therapy, which, in all cases, was performed by HD ().
Table 2. Renal survival (HD dependent/independent) at the end of tandem PE and HD (TPH) treatment according to etiology.
DISCUSSION
PE and HD can be simultaneously performed in patients who require both procedures to treat their underlying disease. TPH procedure saves time and improves dialysis staff/procedure ratio, thus reducing the total cost of the treatment.
In our study, TPH never exceeded 240 min. Total treatment time decreased by at least 90 min compared with the conventional procedures. The longer a procedure is, the more negative impact it has on the patient’s comfort—a patently relevant concern if HD or PE is required daily.
The efficacy of PE has been described extensively. PE has reduced TMA mortality in patients from 94.5% to 13%.Citation9 Many cases of TMA do not develop renal failure that required renal replacement therapy. Three of our patients with TMA needed dialysis, one of whom was dependent on HD at the end of treatment. The patient survival rate in our series was 100%.
In a study by White et al.,Citation11 eight of nine patients with AHR maintained graft function after PE treatment. Two of six of our patients with AHR recovered renal function. The poor results that we observed might be due to the types of patients that we included in our study (all of them were on HD at the start of PE).
In RPGN with crescents, the degree of renal failure at baseline is an important predictor of prognosis.Citation5 All of our patients with RPGN required dialysis at the time of diagnosis. At the end of treatment, 50% of patients no longer required dialysis.
A study by Levy et al.,Citation4 which included 71 patients with antiglomerular basement membrane disease, showed that renal function impairment at admission influenced long-term renal prognosis. We included six patients with Goodpasture’s disease, all of whom required HD at admission. After treatment, one died and five were dependent on HD.
Several studies have demonstrated the safety of PE itself. The rate of complications from PE alone ranges between 4.75% and 9.7%.Citation14,19 Jayne et al.Citation6 compared the adverse effects in patients with vasculitis who were treated with PE versus high-dose intravenous steroids and noted similar rates between groups.
No reports have described the complications that result from combined treatment of the two techniques in detail. Minor adverse events occurred in 10.45% of our cases, with a 0.69% of extracorporeal circuit clotting, and without major events when compared with the results reported by Basic-Jukic et al.Citation19 The hemodynamic stability was excellent, with an incidence of symptomatic hypotension that was even lower than our HD population (3.83%). It has been reported that the use of calcium supplements reduces the incidence of adverse effects.Citation14 Only 0.69% of our 287 sessions showed symptoms of hypocalcemia (paresthesias).
Simultaneous application of PE and HD has been previously reported, but most such studies examined case series, such as Bhowmik et al.,Citation15 which described two isolated cases. Dechmann-Sültemeyer et al.Citation18 recently published an interesting study including 483 simultaneous HD and PE sessions during the past 16 years, describing a technique that reduced overall treatment time without generating deleterious effects on acid–base balance or a substantial increase in adverse reactions. This study showed that the simultaneous technique has clear advantages without creating serious inconveniences. However, we noted some differences in the characteristics of the technique between this study and our trial. TPH reported by these authors was performed with a blood flow rate that ranged from 150 to 200 mL/min. By contrast, in our study, patients were dialyzed at a flow rate of 313 mL/min, allowing the attainment of an adequate dialysis dose without increasing the duration of TPH session. The high blood flow achieved in our schedule design is mainly due to the order in which HD and PE were carried out. In the TPH reported by Dechmann-Sültemeyer et al.,Citation18 they began with PE procedure and in a second step the HD was connected to the PE system. Conversely, we initially started with the HD procedure using a high blood flow and thereafter the PE was connected to the HD system. Our setup allows achieving a higher blood flow rate than the other alternatives previously reported.
Although simultaneous PE and HD is not used in most dialysis units, our experience and that report by Dechmann-Sültemeyer et al.Citation18 with this technique confirm its advantages. In addition, we have introduced simultaneous PE and HD without altering the flow characteristics that are required in each technique.
In conclusion, our results suggest that TPH is an effective treatment that has a good cost–benefit balance. This technique significantly reduces the time of patient exposure to an extracorporeal circuit, decreasing the risks that are associated with anticoagulation agents and optimizing the human resources. Based on the favorable results observed, we strongly recommend this treatment as the most appropriate therapeutic option for patients requiring treatment with HD and PE.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Warren SE, Mitas II JA, Golbus SM, Swerdlin AR, Cohen IM, Cronin RE. Recovery from rapidly progressive glomerulonephritis. Improvement after plasmapheresis and immunosuppression. Arch Intern Med. 1981;141:175–180.
- Grcevska L, Polenaković M, Stojkovski L, Polenaković H. Plasmapheresis in treatment of acute oligoanuria in crescentic glomerulonephritis. Artif Organs. 1998;22:827–830.
- Pusey CD. Anti-glomerular basement membrane disease. Kidney Int. 2003;64:1535–1550.
- Levy JB, Turner AN, Rees AJ, Pusey CD. Long-term outcome of antiglomerular basement membrane antibody treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033–1042.
- Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164–1177.
- Jayne DR, Gaskin G, Rasmussen N, . Randomized trial of plasma exchange or high-dose methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–2188.
- Gaskin G, Pusey CD. Plasmapheresis in antineutrophil cytoplasmic antibody-associated systemic vasculitis. Ther Apher. 2001;5:176–178.
- Rahman T, Harper L. Plasmapheresis in nephrology: An update. Curr Opin Nephrol Hypertens. 2006;15:603–609.
- Von Baeyer H. Plasmapheresis in thrombotic microangiopathy-associated syndromes: Review of outcome data derived from clinical trials and open studies. Ther Apher. 2002;6:320–328.
- Corrigan Jr. JJ, Boineau FG. Hemolytic-uremic syndrome. Pediatr Rev. 2001;22:365–369.
- White NB, Greenstein SM, Cantafio AW, . Successful rescue therapy with plasmapheresis and intravenous immunoglobulin for acute humoral renal transplant rejection. Transplantation. 2004;78:772–774.
- Stegall MD, Gloor JM. Deciphering antibody-mediated rejection: New insights into mechanisms and treatment. Curr Opin Organ Transplant. 2010;15:8–10.
- Gomes AM, Pedroso S, Martins LS, . Diagnosis and treatment of acute humoral kidney allograft rejection. Transplant Proc. 2009;41:855–858.
- Mokrzycki MH, Kaplan AA. Therapeutic plasma exchange: Complications and management. Am J Kidney Dis. 1994;23:817–827.
- Bhowmik D, Jain PK, Maíz JA, . Tandem plasmapheresis and hemodialysis. Ther Apher. 2001;5:439–441.
- Mahmood A, Sodano D, Dash A, . Therapeutic plasma exchange performed in tandem with hemodialysis for patients with M-protein disorders. J Clin Apher. 2006;21:100–104.
- Martínez Gómez A, Tierno Tendero C, Labrid Cantarell M, . Hemodiálisis y plasmaféresis simultáneas. SEDEN No. 3, Volumen 1, III Trimestre 1998.
- Dechmann-Sültemeyer T, Linkeschova R, Lenzen K, Kuril Z, Grabensee B, Voiculescu A. Tandem plasmapheresis and hemodialysis as a safe procedure in 82 patients with immune-mediated disease. Nephrol Dial Transplant. 2009;24:252–257.
- Basic-Jukic N, Kes P, Glavas-Boras S, Brunetta B, Bubic-Filipi L, Puretic Z. Complications of therapeutic plasma exchange: Experience with 4857 treatments. Ther Apher Dial. 2005;9:391–395.