Abstract
Background: Osteoporosis/osteopenia after kidney transplantation is multifactorial, and the mechanism responsible for this condition is unclear. A cumulative steroid dose and female gender are two likely major risk factors for osteoporosis/osteopenia after transplantation, but there is no consensus as to which risk factors are most strongly associated with reduced bone mineral density (BMD). Methods: We assessed 84 kidney recipients who had received transplants at least 5 months prior to enrollment in the study. BMD at the lumbar spine, hip, and femoral neck was evaluated by dual-energy X-ray absorptiometry. We used the average BMD (BMDa), defined as the average of the sum of the lumbar spine, hip, and femoral neck mineral density values, as representative of body BMD. Results: This retrospective study revealed inverse correlations between the BMDa and creatinine level and age at transplant as well as a positive correlation with male gender. Osteoporosis occurred in transplantations where the duration since transplantation was longer. Conclusion: This retrospective study demonstrated that a decrease in BMD, reflecting a bone condition tending toward osteoporosis/osteopenia, is inversely correlated with male gender, creatinine level, and age at transplant in kidney recipients. Nonetheless, the time since transplant is higher in the osteoporosis group than in the osteopenia group.
INTRODUCTION
Patients maintained on dialysis for end-stage renal disease exhibit severe mineral and bone problems. Multiple factors may contribute to the loss of bone mineral density (BMD) in chronic renal failure patients, including the duration of prior chronic renal failure and dialysis, persistent metabolic acidosis, hyperparathyroidism, diabetes mellitus (DM), and smoking.Citation1 Renal transplantation restores defective kidney function in patients with chronic renal disease and is expected to progressively correct established bone lesions. Although transplantation may resolve many of the biochemical imbalances associated with chronic renal failure, such as hyperparathyroidism, the associated steroid and other immunosuppressant therapy cause a continuing damage to bone.Citation2,3 Progressive loss of BMD in trabecular bone often occurs early in renal transplantation.Citation4 Although a cumulative steroid dose and female gender are two likely major risk factors after transplantation,Citation1 there is no consensus as to which risk factors are most strongly associated with reduced BMD.Citation5,6 At present, biochemical markers of bone turnover in the serum or urine are not recommended for diagnosis.Citation7 The World Health Organization (WHO) defines osteoporosis as a lumbar spine (LS), femoral neck (FN), or hip (H) BMD of 2.5 standard deviations (SDs) or more below the mean for healthy young adults, as measured by dual-energy X-ray absorptiometry (DEXA). Osteopenia is defined as a BMD between 1 and 2.5 SDs below the mean.Citation7 In this study, we used the average BMD (BMDa), defined as the average of the sum of the LS, hip, and FN mineral density values, as representative of body BMD. The aim of this retrospective study was to evaluate the osteoporosis factors on BMDa values in renal transplantation recipients.
MATERIALS AND METHODS
Study Population
We enrolled 84 kidney recipients (40 males and 44 females) with transplant durations from 5 months to 274 months. BMD measurements of the LS, left hip, and FN were obtained by DEXA from September 2008 to March 2009. Bone condition was defined by the WHO criteria: osteoporosis was defined as a value of BMD >2.5 SD (T-score) below the young adult mean BMD and osteopenia was defined as a value BMD >1.0–2.5 SD below the young adult mean BMD. In this study, we tried to use the BMDa, defined as the average of the sum of the LS, hip, and FN mineral density values, as representative of body BMD. All subjects had received posttransplantation immunosuppression with prednisolone plus calcineurin inhibitors/sirolimus. Blood samples for serum creatinine (Cr), blood urea nitrogen (BUN), calcium, inorganic phosphate, and uric acid were obtained in a fasting state. DM, smoking frequency, alcohol intake, hepatitis B virus (HBV), hepatitis C virus (HCV), and cytomegalovirus (CMV) infections were assessed via the patients’ medical records. Determinations of 24 h urine total protein were obtained for all patients.
Statistical Analysis
Data are expressed as mean ± SD. The Pearson’s correlation coefficient was employed to evaluate the univariate association between the BMDa and the studied variables. Stepwise multiple linear regression analysis was performed to determine which variables were independently correlated with BMDa, LS-BMD, H-BMD, and FN-BMD. For the purpose of this analysis, the dependent variables were BMDa, LS-BMD, H-BMD, and FN-BMD; and the independent variables were age, age at transplantation, time since transplant, Cr, serum uric acid, serum calcium, inorganic phosphate concentrations, male gender, smoking, alcohol consumption, and status of DM, CMV, HBV, and HCV infections. Intergroup comparisons were performed with the Student’s t-test. A least significant difference one-way analysis of variance (ANOVA) was performed to compare the transplantation duration values of the various BMD types. The level of statistical significance was set at p < 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) Version 12.0 for Windows (SPSS, Inc., Chicago, IL, USA).
RESULTS
Patient Characteristics
Of the 84 subjects enrolled in this study, 13 patients had a medical history of DM, 10 patients had HBV infection, 19 patients had HCV infection, and 16 patients had CMV infection. Moreover, 12 males were habitual users of tobacco, and 8 males and 1 female regularly consumed alcohol. lists the characteristics of the study subjects. The BMDa did not differ between DM and non-DM (0.803 ± 0.12 vs. 0.797 ± 0.118, p = 0.865) but showed a significant increase in male subjects compared to female subjects (0.841 ± 0.127 vs. 0.751 ± 0.093, p < 0.001). The mean age of subjects was 50.6 ± 8.8 years. The mean BMDa was 0.79 ± 0.12 g/cm2. The BMDa was negatively correlated with the 24 h urine total protein level, but this was not statistically significant.
Table 1. Characteristics of the kidney recipients.
Table 2. Coefficients of correlation in the Pearson’s correlation analysis between BMDa and other clinical variables.
Table 3. Results of stepwise multiple regression analysis to assess the correlation of BMDa, LS-BMD, H-BMD, and FN-BMD with other variables.
BMDa and Other Clinical Parameters
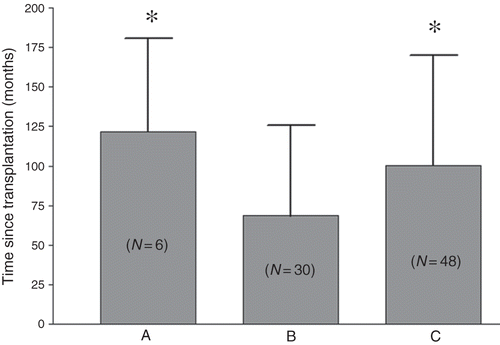
lists the coefficients of correlation in the Pearson’s correlation analysis between BMDa and other clinical variables. This analysis demonstrated that gender and BUN concentration correlated with BMDa. In a stepwise multiple linear regression analysis, we used the BMDa value, LS-BMD, H-BMD, and FN-BMD as the dependent variables, while using age, age at transplantation, time since transplant, Cr, serum uric acid, serum calcium, inorganic phosphate concentrations, male gender, smoking, alcohol consumption, and the status of DM, CMV, HBV, and HCV infections as independent variables. shows the results of the stepwise multiple linear regression analysis between the representations of different BMD and the other clinical variables. Gender, Cr concentration, and age at transplantation were significant variables for BMDa. To further examine the possible influence of clinical features on bone condition, a one-way ANOVA was performed using the WHO criteria as an independent factor. In this study, the normal group contained 6 patients (time since transplant, 121.8 ± 58.7 months), the osteopenia group contained 30 patients (time since transplant, 68.6 ± 57.3 months), and the osteoporosis group contained 48 patients (time since transplant, 100.3 ± 69.7 months). shows the clinical parameters of recipients between normal, osteopenia, and osteoporosis groups. illustrates the difference in time since transplantation among the osteopenia and osteoporosis groups.
Table 4. Clinical parameters of recipients between normal, osteopenia, and osteoporosis groups.
DISCUSSION
High bone mass loss after transplantation occurs when the patient already has a poor-quality skeleton and is additionally influenced by a number of factors, including iatrogenic ones.Citation8 Most of the available information, including that on biochemical markers, has been obtained from patients without kidney disease, and the use of biochemical markers in the assessment of skeletal problems is extremely limitedCitation8; this study examined the feasibility of using the BMDa value for assessing the risk of osteoporosis/osteopenia and viral infection and as an indicator of daily urine total protein in kidney recipients. Our study is the first to demonstrate that the BMDa value is independently positively correlated with the male gender and negatively correlated with renal function and the age at transplant in kidney recipients.
Figure 1. Significant difference of time since transplant in patients with different bone conditions: A, normal; B, osteopenia; C, osteoporosis.
Notes: The number in parentheses indicates the number of patients in each group.
*p < 0.05 versus group B.
An increase in bone mass loss is multifactorial and is affected by age,Citation9 female gender,Citation9–11 renal function, and duration of dialysis before transplantation.Citation11 A major influencing and well-known factor causing increased loss of bone mass is high-dose steroid therapy during the early period after transplantation and continuous long-term steroid administration.Citation3 Bone mass loss in kidney recipients is a result of a combination of increased bone resorption and decreased bone formation, but the precise mechanisms involved are unknown.
Renal transplantation can be associated with dramatically reduced bone mass, the first 3–6 months after transplantation being the most critical period.Citation5,12,13 In a long-term study, the ongoing accelerated lumbar bone mass loss was approximately 1.7% ± 2.8% per year.Citation14 Earlier prospective studies in glucocorticoid-induced osteoporosis suggested more rapid bone loss in the early weeks of steroid therapy that subsequently slowed in later weeks.Citation15 In a cross-sectional study, the time since the transplant was shown to have a high correlation with the cumulative prednisolone dose.Citation2 Julian et al.Citation5 documented a rapid decrease (6.8%) in lumbar BMD in the first 6 months after transplantation, with a slower decrease of 2.6% in the following 12 months. Horber et al.Citation12 reported a similar rapid decrease in lumbar bone mass during the first 5 months after renal transplantation. These studies explain why the time since transplant is longer in the osteoporosis group (n = 48, 100.3 ± 69.7 months) than in the osteopenia group (n = 30, 68.6 ± 57.3 months), as shown in .
Age is a risk factor for osteoporosis, and women older than 65 years and men older than 70 years are recommended for osteoporosis screening in the general population.Citation7 In this study, 92% of the study population (mean age, 51 ± 9 years) had osteopenia/osteoporosis resulting due to prior renal failure and posttransplant iatrogenic effects. Hung et al.Citation9 reported that age was one of the factors for osteoporosis in kidney recipients; however, other studiesCitation2,16 noted that the age of the kidney recipient was not a factor for osteoporosis. From these studies, it appears that the role of age in osteoporosis in kidney recipients is controversial. To our knowledge, the role of age at transplant has been less discussed with respect to osteoporosis in kidney recipients. In normal and in the kidney recipient population between the age of 20 and 70 years, the average LS-BMD and FN-BMD values are probably negatively correlated with age. It is interesting that 12 months following cardiac transplantation, the LS-BMD value was restored to the BMD value at the time of transplant.Citation17 Twenty-four months after transplantation, the yearly loss of absolute BMD was parallel to the age-dependent physiological decline in absolute BMD,Citation18 providing an explanation for why the age at transplant is an important factor determining bone condition of kidney recipients, as shown in our study.
Our study suffered from certain limitations, including retrospective observations, a lack of cumulative steroid and other immunosuppressant agent dosing, and so on. The effect of steroids on bone density has been shown in most studies investigating bone loss after kidney transplantation.Citation3 However, the role of calcineurin inhibitors on bone density is controversial,Citation2,8,11 and at least one study has shown that cyclosporine might have a bone protective effect.Citation19
In conclusion, despite the effect of immunosuppressant agents, this retrospective study has demonstrated that the BMDa value, reflecting bone condition, is positively correlated with the male gender and negatively correlated with the Cr value and the age at transplant in kidney recipients. Nonetheless, the time since transplant is higher in osteoporosis group than in osteopenia group. The negative association between BMDa and age at transplant in kidney recipients is interesting and warrants further investigation of the outcomes of kidney transplants in younger patients.
ACKNOWLEDGMENT
We thank the members of the Immune-Transplant Center in Chang Gung Memorial Hospital for their invaluable and dedicated assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Braun WE. The medical management of the renal transplant recipient. In: Johnson RJ, Fehally J, eds. Comprehensive Clinical Nephrology. London: Mosby; 2003:1118.
- Parker CR, Freemont AJ, Blackwell PJ, Grainge MJ, Hosking DJ. Cross-sectional analysis of renal transplantation osteoporosis. J Bone Miner Res. 1999;14(11):1943–1951.
- Kodras K, Haas M. Effect of kidney transplantation on bone. Eur J Clin Invest. 2006;36(Suppl. 2):63–75.
- Rodino MA, Shane E. Osteoporosis after organ transplantation. Am J Med. 1998;104(5):459–469.
- Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325(8):544–550.
- Grotz WH, Mundinger FA, Rasenack J, . Bone loss after kidney transplantation: A longitudinal study in 115 graft recipients. Nephrol Dial Transplant. 1995;10(11):2096–2100.
- Sweet MG, Sweet JM, Jeremiah MP, Galazka SS. Diagnosis and treatment of osteoporosis. Am Fam Physician. 2009;79(3):193–200.
- Cunningham J. Posttransplantation bone disease. Transplantation. 2005;79(6):629–634.
- Hung CJ, Lee PC, Song CM, . Clinical implication of hormone treatment in postmenopausal kidney transplants. Transplant Proc. 1996;28(3):1548–1550.
- Wolpaw T, Deal CL, Fleming-Brooks S, Bartucci MR, Schulak JA, Hricik DE. Factors influencing vertebral bone density after renal transplantation. Transplantation. 1994;58(11):1186–1189.
- Aroldi A, Tarantino A, Montagnino G, Cesana B, Cocucci C, Ponticelli C. Effects of three immunosuppressive regimens on vertebral bone density in renal transplant recipients: A prospective study. Transplantation. 1997;63(3):380–386.
- Horber FF, Casez JP, Steiger U, Czerniak A, Montandon A, Jaeger P. Changes in bone mass early after kidney transplantation. J Bone Miner Res. 1994;9(1):1–9.
- Ezaitouni F, Westeel PF, Fardellone P, . Long-term stability of bone mineral density in patients with renal transplant treated with cyclosporine and low doses of corticoids. Protective role of cyclosporine? Presse Med. 1998;27(15):705–712.
- Pichette V, Bonnardeaux A, Prudhomme L, Gagné M, Cardinal J, Ouimet D. Long-term bone loss in kidney transplant recipients: A cross-sectional and longitudinal study. Am J Kidney Dis. 1996;28(1):105–114.
- Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: Pathogenesis and management. Ann Intern Med. 1990;112(5):352–364.
- Durieux S, Mercadal L, Orcel P, . Bone mineral density and fracture prevalence in long-term kidney graft recipients. Transplantation. 2002;74(4):496–500.
- Leidig-Bruckner G, Hosch S, Dodidou P, . Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: A follow-up study. Lancet. 2001;357(9253):342–347.
- Grotz WH, Mundinger FA, Gugel B, Exner VM, Kirste G, Schollmeyer PJ. Bone mineral density after kidney transplantation. A cross-sectional study in 190 graft recipients up to 20 years after transplantation. Transplantation. 1995;59(7):982–986.
- Carlini RG, Rojas E, Weisinger JR, . Bone disease in patients with long-term renal transplantation and normal renal function. Am J Kidney Dis. 2000;36(1):160–166.