Abstract
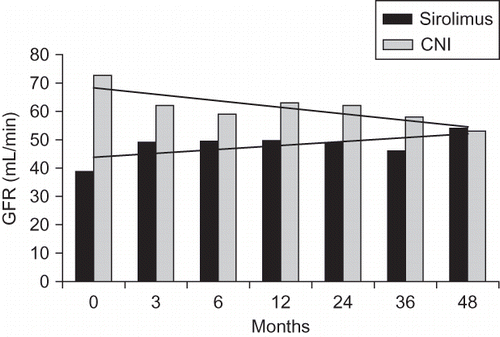
In this retrospective study, 83 patients were accepted. Mammalian target of rapamycin (mTOR) group consisting of 37 patients were converted from calcineurin inhibitors (CNI), and the control group included 46 patients (initially CNI-receiving patients). As a control-match of each mTOR inhibitor patient, the succeeding patient with transplantation who continued CNI therapy was chosen. All patients received CNI, MMF, and prednisolone as an immunosuppressive therapy initially. In comparison of two groups, there was no significant difference between sex, donor organ source, donor organ ischemia time, or mismatches. However, mean age between groups was significantly different (mTOR group: 48.3 ± 12, CNI group: 38.6 ± 11, p < 0.001). Decision of conversion to mTOR inhibitors in 30 patients was made by biopsy. The reasons for conversion were determined as CNI nephrotoxicity in 15 patients, chronic allograft nephropathy in 15 patients, malignancy in 6 patients, and renal artery stenosis in 1 patient. Basal glomerular filtration rates (GFRs) were markedly lower in mTOR group than in CNI group (38.8 mL/min vs. 72.7 mL/min). At the end of 48-month follow-ups, GFR increased from 38 mL/min to 54 mL/min in mTOR group; however, it decreased to 53 mL/min from 72 mL/min in CNI group. There was no difference left between the two groups in GFR after 4-year follow-up. Hyperlipidemia was higher in mTOR group. Acute rejection rates were similar. Cytomegalovirus (CMV) disease was more prevalent in CNI group. Graft failure developed due to secondary reasons, causing mortality in both groups. We suggest that conversion to mTOR inhibitors maintains and improves graft functions well.
INTRODUCTION
Calcineurin inhibitors (CNI) are one of the main immunosuppressive agents in renal transplantation since introduction.Citation1 CNI increase short-time graft survival significantly but no improvements in long-term survival is detected. Chronic allograft nephropathy (CAN) is a main problem that affects long-term outcome in patients.Citation2–4 Etiology of CAN is classified as immune-dependent or non-immune-dependent. Immune-dependent reasons are defined as acute or chronic, cellular, and antibody-mediated response to donor antigens. On the other hand, characteristics of donor, cold ischemia time, cytomegalovirus (CMV) infections, hypertension, hyperlipidemia, and, most importantly, CNI are major non-immune-dependent reasons. CAN is proved in 72% of biopsy series in second year of transplantation.Citation5 The features of CAN are detected clearly even in patients with stable and even optimal renal functions.Citation6
CNI cause dose-related and reversible renal vasoconstriction and decrease in glomerular filtration rate (GFR) in short-term period compared with chronic and irreversible interstitial fibrosis and minimal thickening related to arteriolar intimal fibrosis in long term.Citation7 By immunosuppressive regimens without CNI, observed decrease in development and progression of CAN contributes to long-term survival. Therefore it is recommended that the use of mammalian target of rapamycin (mTOR) inhibitors may prevent and treat CAN. The beneficial effects were shown with the combination therapy of CNI and mTOR inhibitors for 3 months and then CNI was withdrawn.Citation8 Late conversion to mTOR inhibitors also appears to be efficacious regarding CAN.Citation9 mTOR inhibitors inhibit the smooth muscle cell and fibroblast proliferation in vitro.Citation10 On top of all this, sirolimus has been shown to decrease in intimal hyperplasia in immune and non-immune vascular injury models.Citation11 Considering positive effects, mTOR inhibitors can be beneficial in CAN.
In this study, we aimed to analyze the results between conversion from CNI to mTOR inhibitors and CNI continuation in kidney recipients for 4 years.
MATERIALS AND METHOD
Study Design and Patients
Eighty-three patients attended the study. The 46 patients in mTOR study group included the 37 patients converted from CNI. Conversion group was using cyclosporine therapy. Immunosuppressive therapy was converted from CNI to mTORs in 30 patients because of biopsy-proven calcineurin toxicity (15 patients), and CAN in others. Six patients had malignancy (two epidermal squamous cell cancer, one Kaposi’s sarcoma, one Hodgkin’s disease, one renal cell cancer in native kidney, and one thyroid papillary cancer), and one patient had renal artery stenosis. Since we perform renal biopsies to the patients only when graft functions deteriorate, we had no biopsies in the calcineurin group. All patients converted from CNI were beyond 6 months posttransplantation. And all had progressive deterioration in graft functions, which is defined as an increase of creatinine more than 25% in 6 months.
As a control-match of each mTOR inhibitor patient, the succeeding patient with transplantation who continued CNI therapy was chosen. Control group included 46 patients.
We obtained data through records of each patient regarding age; sex; donor age; mismatch; donor organ source; cold ischemia time; induction therapy; acute rejection time and frequency; time up to conversion; immunosuppressive treatment up to conversion; CNI trough levels; and hypertension, creatinine, GFR, aspartate aminotransferase (AST), alanine aminotransferase (ALT), hemoglobin, leukocyte, platelet, triglyceride, low-density lipoprotein (LDL)-cholesterol, and the level of proteinuria in 24-h urine at the conversion time. Also occurrence of hypertension, creatinine levels, GFR, AST, ALT, hemoglobin, leukocyte, platelet, triglyceride, LDL-cholesterol values, and proteinuria in 24-h urine at 3, 6, 12, 24, 36, and 48 months after conversion were recorded.
Exclusion criteria included patients who required dialysis, those with acute rejection attacks during last 3 months, those with more than 800 mg/day proteinuria, those with recurrent renal disease, and those who had leukopenia (leukocyte counts less than 4 × 109/L) and/or thrombocytopenia (platelet counts less than 100 × 109/L). Patients with retransplantation were also excluded. The pregnancy test should be negative.
Conversion Protocol
CNI and mycophenolate mofetil/mycophenolate sodium or azathioprine and prednisolone were prescribed for all patients as immunosuppressive protocol. After conversion dosages of mycophenolate mofetil and prednisolone were not changed. On conversion from CNI to sirolimus, patients took their last CNI dose at night and then continued with sirolimus dose of 5 mg/day for the first 3 days. Sirolimus doses were reduced to 3 mg/day in consequent days. The blood levels of sirolimus were monitored for dosage adjustments to achieve concentrations of 5–8 ng/mL. The sirolimus group had mean dosage of 2.56 mg/day and trough levels of 5.72 ng/mL. The calcineurin group had mean dosage of 227 mg/day and trough levels (C2) of 726 ng/mL. The drug dosages have been modified according to trough levels.
Follow-Up Criteria
Graft and patient survival, calculated GFRs (at 3, 6, 12, 24, 36, and 48 months) by Modification of Diet in Renal Disease (MDRD), GFR alternation, acute rejection rates, frequency of CMV and/or BK viruses (Polyoma viruses) infections, new-onset hypertension (described as ≥140/90 mmHg), hyperlipidemia (triglyceride ≥300 mg/dL or LDL ≥ 160 mg/dL), proteinuria (≥1 g/day), transaminase, and hematologic data (anemia, leukopenia, and thrombocytopenia) were noted for both groups.
STATISTICS
Continuous variables are expressed as mean ± SD. To evaluate the difference between mean values Student’s t-test was employed. Chi-square test was used for categoric variables, and p ≤ 0.05 was accepted as significant. The major statistics were calculated on a computer using SPSS 15.0 (Statistical Package for the Social Sciences, Inc., Chicago, IL, USA).
RESULTS
Demographic Characteristics of Patients
shows the initial characteristics of patients. At the time of conversion, patients in the sirolimus group were older than in CNI group (48.3 vs. 38.6, p = 0.001). There was no difference with respect to sex, donor age, mismatches, cold ischemia time, donor organ source, duration of CNI use, and duration of dialysis period prior to transplantation between groups. As an induction therapy antithymocyte globulin (ATG) was used more in sirolimus group (18 vs. 14) than in CNI group to whom basiliximab was given more (23 vs. 12). The mean duration from transplantation to conversion was 30.2 (6–108) months. At the time of conversion, the GFR calculated by MDRD formula was significantly lower in sirolimus group (38.8 vs. 72.7, p <0.001).
Table 1. Initial characteristics of patients.
Graft and Patient Survival
Patient survival in sirolimus group at 48 months after conversion was 91.9% (34 out of 37) and in CNI group 87% (40 out of 46). Deaths were due to sepsis in three patients, myocardial infarction in two patients, intracerebral bleeding in one patient in CNI group and due to sepsis in two patients and ischemic cerebrovascular infarct in one patient in mTOR group. All patients who died had functional grafts. Two patients of CNI group who had transplantation 7 and 8 years ago, respectively, and one patient transplanted 8 years ago in sirolimus group returned to dialysis because of graft dysfunction.
Graft Functions
At the time of conversion the level of serum creatinine in sirolimus group was 1.89 mg/dL and GFR was 38.8 mL/min. In CNI group serum creatinine was 1.32 mg/dL and GFR was 72.7 mL/min (p < 0.001). Through follow-up, GFR was shown to decrease in CNI group while it increased in sirolimus group (). At the end of 48 months there was no difference with respect to GFR levels between the groups ().
Table 2. GFR levels at months 3, 6, 12, 24, 36, and 48.
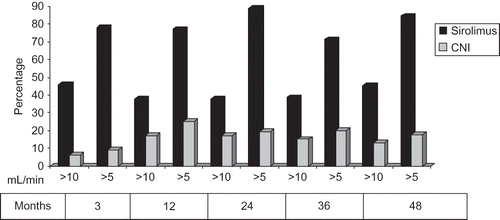
In the increases of GFR ≥ 5 and ≥ 10 mL/min at 48 months of therapy are shown. There is significantly higher increase of GFRs (of 5 mL/min and 10 mL/min, respectively) in sirolimus group at 48th month compared with that of CNI group (84.6%/45.1% vs. 17.6%/13.1%, respectively) (p < 0.05).
Figure 2. Percentage of patients showing clinically meaningful improvement in GFR at 3, 12, 24, 36, and 48 months.
Notes: p < 0.05; GFR, glomerular filtration rate; CNI, calcineurin inhibitors.
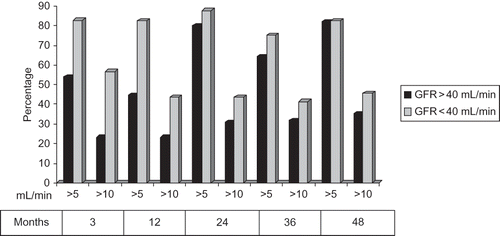
The patients in sirolimus group with GFRs over 40 mL/min and those with less than 40 mL/min were compared to detect the improvements of GFR (≥ 5 mL/min and ≥10 mL/min) at 48th month; ≥10 mL/min increase of GFR in patients with GFR <40 mL/min was observed in 45.4% of patients and in 35.3% of patients with GFR ≥ 40 mL/min (p = 0.064) ().
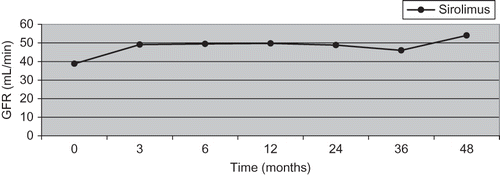
Figure 3. GFR changes in sirolimus group at 3, 12, 24, 36, and 48 months.
Notes: p < 0.05; GFR, glomerular filtration rate; CNI, calcineurin inhibitors.
The increase in GFR in 3 months in sirolimus group was observed to last till the 48th month ().
Adverse Effects
Acute rejection was not detected at 3 months. Acute cellular rejection-type IA occurred in one patient in sirolimus group at 6th month and in one patient at 12th month. There was no graft failure in patients who had rejection attacks. Thirteen patients had CMV infection in CNI group compared with 5 patients in sirolimus group (0.046). Hyperlipidemia was found significantly higher in sirolimus group (64.9%, 24 patients vs. 23.9%, 11 patients, p < 0.001). The frequency of oral aphthous stomatitis and of BK virus nephropathy were not different between groups. Proteinuria was more frequent in sirolimus group, but not statistically significant (5 vs. 3, p = 0.29). Three patients had proteinuria in nephrotic range in sirolimus group, two patients were withdrawn of sirolimus therapy, and one was controlled by renin angiotensin aldosterone system (RAAS) blockade.
DISCUSSION
CAN and CNI toxicity can cause graft function failure. Although acute rejection in the first year after transplantation rates was 5–20% with CNI regimens, CNI nephrotoxicity and CAN remain the major problems in long-term graft survival.Citation2–4 The most important contributing factor to CAN is calcineurin toxicity. In this setting the most frequently used rescue regimen is conversion to sirolimus from CNI.Citation12
In this study, we observed improvement in graft functions after 3 years of renal transplantation in patients converted to sirolimus from CNI. There was significant improvement in GFR after 3 months of conversion, which continued for 48 months even in patients with GFR below 40 mL/min. A similar improvement proved to be in biopsy-proven CNI nephrotoxicity and CAN patients, diagnosed before conversion. There was a failure of improvement in GFR in CNI group. Improvement and maintenance of GFR through 48 months in the majority of patients might be explained by the absence of proteinuria at time point of conversion and by low CAN scores that our patients had.
In this study, it is shown that conversion to sirolimus increased GFR even when the initial graft function is impaired. There is decreased GFR in the control group that used CNI as the same period of time as the converted patients. There is no difference with respect to GFR between mTOR and CNI groups at 48th month. There is ample evidence in the literature about improvement in graft function after conversion to sirolimus. However, this is the first study proving achievement of better graft functions in sirolimus-converted patients that had significant graft dysfunction compared with CNI group that initially has well-functioning grafts.Citation5,13,14 Citterlo et al.Citation14 declared that the patients diagnosed with CAN would improve in renal function by 57% on conversion from CNI to sirolimus. Egidi et al.Citation15 observed 11 mL/min increase in GFR after sirolimus conversion. Diekmann et al.Citation13 announced that after conversion to sirolimus, 54% of CAN patients’ graft functions remain stable or improved. The predictor of successful conversion was presence or absence of proteinuria at the time of conversion (not creatinine).
In our study similar to earlier evidence hyperlipidemia was observed in 64.9% of sirolimus group.Citation12 It was controlled by statin treatment. Proteinuria was not significantly higher in sirolimus group. Two patients were withdrawn from sirolimus therapy because of nephrotic range proteinuria that was unresponsive to RAAS blockade. The reason for higher proteinuria in sirolimus group can be attributed to defective graft functions or withdrawal from cyclosporine therapy.Citation16,17 We did not observe any increase in acute rejection rates after sirolimus conversion as mentioned in earlier studies.Citation18 The frequency of acute rejection is not increased in our study. It may be because conversion was made at 30th month.
Liver function tests and hematologic parameters remained stable. Increased rate of CMV infection in CNI group could be because of immune oversuppression, although the level of CNI was in therapeutic range.
In conclusion, in this study we proved that graft survival improved by conversion from CNI to sirolimus in patients with graft dysfunctions. Although the study group was small, we suggest that conversion to mTOR inhibitors in appropriate patients maintains and even improves graft functions well.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Calne RY, Rolles K, White DJ, . Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036.
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612.
- Rasucen LC, Solez K, Colvin R. Fibrosis and atrophy in the renal allograft: Interim report and new directions. Am J Transplant. 2002;2:203–206.
- Pascula M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346:580–590.
- Ram Peddi V, Jensik S, Pescovitz M, . An open-label, pilot study evaluating the safety and efficacy of converting from calcineurin inhibitors to sirolimus in established renal allograft recipients with moderate renal insufficiency. Clin Transplant. 2005;19:130–136.
- Seron D, Moreso F, Fulladosa X, Hueso M, Carrera M, Grinyo JM. Reliability of chronic allograft nephropathy diagnosis in sequential protocol biopsies. Kidney Int. 2002;61:727–734.
- Schrama YC, Joles JA, van Tol A, Boer P, Koomans HA, Hene RJ. Conversion to mycophenolate mofetil in conjunction with stepwise withdrawal of cyclosporine in stable renal transplant recipients. Transplantation. 2000;69:376–383.
- Kreis H, Oberbauer R, Campistol JM, . Rapamycinmune maintenance regimen trial. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004;15:809–817.
- Weber T, Abendroth D, Schelzig H. Rapamycin rescue therapy in patients after kidney transplantation: First clinical experience. Transpl Int. 2005;18(2):151–156.
- Morris RE, Cao W, Huang X, . Rapamycin (sirolimus) inhibits vascular smooth muscle DNA synthesis in vitro and suppresses narrowing in arterial allografts and ballooninjured carotid arteries: Evidence that rapamycin antagonizes growth factor action on immune and nonimmune cells. Transplant Proc. 1995;27:430–431.
- Suzuki T, Kopia G, Hayashi S, . Stentbased delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001;104:1188–1194.
- Sayin B, Karakayali H, Colak T, . Conversion to sirolimus for chronic allograft nephropathy and calcineurin inhibitor toxicity and adverse effect of sirolimus after conversion. Transplant Proc. 2009;41:2789–2793.
- Diekmann F, Budde K, Oppenheimer F, Fritsche L, Neumayer HH, Campistol JM. Predictors of success in conversion from calcineurin inhibitor to sirolimus in chronic allograft dysfunction. Am J Transplant. 1875;2004(4):1869–1875.
- Citterlo F, Scata MC, Violi P, . Rapid conversion to sirolimus for chronic progressive deterioration of the renal function in kidney allograft recipients. Transplant Proc. 2003;35:1292–1294.
- Egidi MF, Cowan PA, Naseer A, Gaber AO. Conversion to sirolimus in solid organ transplantation: A single-center experience. Tranplant Proc. 2003;35(Suppl. 3):131–137.
- Schena FP, Pascoe MD, Alberu J, . Sirolimus CONVERT Trial Study Group. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation. 2009;87(2):233–242.
- Boratynska M, Banasik M, Watorek E, . Conversion to sirolimus from cyclosporine may induce nephrotic proteinuria and progressive deterioration of renal function in chronic allograft nephropathy patients. Transplant Proc. 2006;38(101):104.
- Smak Gregoor PJ, de Sevaux RG, Ligtenberg G, . Withdrawal of cyclosporine or prednisone six months after kidney transplantation in patients on triple drug therapy: A randomized, prospective, multicenter study. J Am Soc Nephrol. 2002;13:1365–1373.