Abstract
Renal ischemia/reperfusion (I/R) injury is a major cause of renal failure. The aim of our study is to explore the role of lysophosphatidic acid (LPA) and lovastatin on renal I/R injury and its mechanism in the rat. Male Wistar rats were randomly divided into sham-operated group; renal I/R for 0 h, 4 h, 12 h, and 24 h groups; LPA treatment group; and lovastatin treatment group (n = 10). Rats were killed to determine the level of monocyte chemotactic protein-1 (MCP-1) in renal tissue, renal function [serum creatinine (Cr) and blood urea nitrogen (BUN)], and renal histomorphology to evaluate the effectiveness of LPA and lovastatin. Normal renal tissue had a low level of MCP-1. The level of MCP-1 began to rise at 0 h after reperfusion, reached peak value at 4 h, and then gradually fell off. Compared with sham-operated group, MCP-1 was increased in all renal I/R injury groups (p < 0.01). With the extension of reperfusion, Cr and BUN were significantly increased (p < 0.01). There were damages in kidney tubules, renal interstitium, and kidney glomerulus in renal I/R injury groups. Paller’s score was significantly increased in all renal I/R injury groups compared with sham-operated group (p < 0.01). LPA and lovastatin reduced the level of MCP-1, Cr, BUN, and damages of renal histomorphology (p < 0.01). The level of MCP-1 in renal tissue dynamically increases in renal I/R injury, indicating that MCP-1 is involved in renal I/R injury. LPA and lovastatin might protect renal function by downregulating MCP-1 in renal I/R injury.
INTRODUCTION
Renal ischemia/reperfusion (I/R) injury is a problem in clinical practice commonly observed after transplantation, cardiac and vascular surgery, shock, and trauma.Citation1 The damage of renal I/R injury is partially due to infiltration of inflammatory cells which releases a series of cytokine/enzymes and leads to inflammation and tissue damage. Cell apoptosis and necrosis occur during renal I/R injury. At the same time, inflammatory responses are also induced by renal I/R injury, which contains activating complement system and inducing continuous inflow of cytokines, chemotactic factors, and neutrophilic granulocytes.
The chemotactic factor belongs to cytokine superfamily. According to the arrangement of cysteine, chemotactic factors are divided into CXC, CC, C, and CX3C families.Citation2 Monocyte chemotactic protein (MCP) includes MCP-1, 2, and 3, and MCP-1 (also known as CCL2) belongs to CC family. Human MCP-1 may facilitate mononuclear macrophage infiltration into renal tissue and induce increase of Ca2+ in monocytes/macrophages, which causes respiratory burst, lysosome release, and oxygen-derived free radicals’ production to lead to renal damage.Citation3–6 MCP-1 has chemotactic and activated effects on mononuclear macrophage both in vitro and in vivo. In human normal renal tissue, the level of MCP-1 is low. Animal experiment and clinical observations have indicated that under the conditions of ischemia, hypoxia, intoxication, inflammation, and allergic reaction, expression of MCP-1 is elevated.Citation7,8 MCP-1/CCR2 signaling contributes to the pathogenesis of renal I/R injury,Citation9 and blockage of the signaling rescues renal I/R injury.Citation10 In rat renal I/R injury, cytokine KC and MIP-2 are also upregulated.Citation11,12
Studies have been performed to prevent the renal I/R injury. Molecules and peptide were reported to have protective effect on renal function in I/R injury.Citation13–15 Mesenchymal stem cells and human hematopoietic stem/progenitor cells were used to treat rat renal I/R injury, which facilitates repairing of renal tissue.Citation16–18 It has been demonstrated that lysophosphatidic acid (LPA) may protect renal function by anti-inflammatory, inhibiting expression of tumor necrosis factor, inflow of neutrophilic granulocyte, and activation of complement.Citation11 However, LPA does not have influence on high level of cytokine KC and MIP-2 induced by rat renal I/R injury.Citation11 Additionally, lovastatin is a classical and effective lipid-lowering drug and also has protective effect on kidney.Citation19,20 Little is known about the mechanism. The purpose of the present study was to investigate the role of LPA and lovastatin on renal I/R injury in rat and its mechanism.
MATERIALS AND METHODS
Animals and Treatment
Eight-week-old male Wistar rats, weighing 230 ± 20 g, were provided by Laboratory Animal Center, Inner Mongolia University. The rats were maintained at room temperature and fed with standard diet and clean water. All animal experiments were approved by Animal Care and Use Committee, Inner Mongolia Medical College.
Seventy Wistar rats were randomly divided into seven groups (n = 10) including sham-operated group; renal post-I/R for 0 h, 4 h, 12 h, and 24 h groups; LPA treatment group; and lovastatin treatment group. Before operation, all the rats were anesthetized with intraperitoneal injection of 3% sodium pentobarbital (50 mg/kg). The right kidney was removed. Ischemia was induced by clamping the left renal artery for 60 min.Citation21 Rats were intraperitoneally injected phosphate-buffered saline (PBS) (1 mL/rat) before reperfusion and sacrificed after reperfusion for 0 h, 4 h, 12 h, and 24 h. In sham-operated group, rats were sacrificed 5 h after sham operation. During ischemia, rats were intraperitoneally injected 1 mg LPA (Sigma, St. Louis, MO, USA) dissolved in 1 mL PBS and killed after reperfusion for 4 h. In lovastatin group, rats were treated with lovastatin (2 mg/kg day, H10970092, Winsunny, Beijing, China) by oral administration for 3 days before operation, and then the rats were killed after reperfusion for 4 h. Two milliliters of blood and the left kidney were harvested from each rat for further analysis.
ELISA
The concentration of MCP-1 was determined by MCP/CCL2 Rat ELISA kit (SUNBIO, Beijing, China) according to the manufacturer’s manual.
Renal Functional Test
Serum creatinine (Cr) and blood urea nitrogen (BUN) in blood were determined by automatic biochemical analyzer.
Paller’s Score
Renal tissue was embedded in paraffin. The H&E staining in standard procedure was performed in the sectioned tissues (4 μm thick). Tissue sections with clear cell morphology and structure were selected and observed under light microscope (×400). Five fields were randomly selected in each section (a total of 50 kidney tubules) to assess the scores according to Paller’s method.Citation22 That is, obvious expansion of kidney tubules and flat cells are regarded as one score; brush border injury or desquamation as one or two scores; cast as two scores; and deciduous necrotic cells, but no cast or cell debris as one score.
Statistical Analysis
Statistical analysis was performed with SPSS (NY, USA) 13.0 software. Experimental data were expressed as mean ± SD. The t-test was used for comparison between two groups. The one-factor analysis of variance was used for the comparison between multiple groups; the q-test was used in multiple comparisons which indicated statistical significance by the F-test. A p-value of <0.05 was considered significant.
RESULTS
Level of MCP-1 in Renal I/R Injury Rat
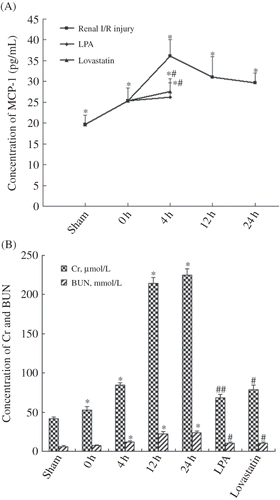
Compared with sham-operated group, expression of MCP-1 was elevated in all I/R injury groups (p < 0.01). The level of MCP-1 began to rise at 0 h after reperfusion, reached peak value at 4 h, and then gradually fell off. The level of MCP-1 at 24 h was still higher than it was at 0 h (29.17 ± 2.25 pg/mL vs. 25.38 ± 3.04 pg/mL, A). One-factor analysis of variance indicated that there was significant difference of MCP-1 level in all I/R injury groups compared to sham-operated group. The level of MCP-1 in renal tissue decreased in LPA and lovastatin treatment groups compared with 4 h I/R group (26.19 ± 3.46 pg/mL, 27.58 ± 3.05 pg/mL vs. 36.05 ± 4.04 pg/mL, p < 0.01, A).
Figure 1. (A) Concentration of monocyte chemotactic protein-1 (MCP-1) in renal tissue from sham-operated group, ischemia/reperfusion 0 h, 4 h, 12 h, and 24 h groups, lysophosphatidic acid (LPA) treatment group, and lovastatin treatment group. During ischemia, rats were intraperitoneally injected 1 mg LPA dissolved in 1 mL phosphate-buffered saline and killed after reperfusion for 4 h. In lovastatin group, rats were treated with lovastatin by oral administration for 3 days before operation, and the rats were killed after reperfusion for 4 h. *p < 0.01, compared with sham-operated group. **p < 0.01, compared with ischemia/reperfusion 4 h group. (B) The level of serum creatinine (Cr) and blood urea nitrogen (BUN) in blood from sham-operated group, ischemia/reperfusion 0 h, 4 h, 12 h, and 24 h groups, LPA treatment group, and lovastatin treatment group. *p < 0.01, compared with sham-operated group. **p < 0.05, ***p < 0.01 compared with ischemia/reperfusion 4 h group.
Renal Functional Change in Renal I/R Injury Rat
Cr and BUN were measured for renal function. With the extension of reperfusion, Cr and BUN gradually increased. Compared with sham-operated group, the levels of Cr and BUN in all I/R injury groups were significantly increased (p < 0.01, B). One-factor analysis of variance indicated that Cr level of each I/R injury group was significantly higher than in sham-operated group, and BUN level of 4 h, 12 h, and 24 h I/R groups was significantly higher than in sham-operated group. Compared with 4 h I/R group, levels of Cr and BUN were all decreased in LPA and lovastatin treatment groups by 18.6% and 6.3% (68.53 ± 4.46 μmol/L, 78.88 ± 5.39 μmol/L vs. 84.22 ± 3.61 μmol/L, p < 0.01, p < 0.05) and 13.2% and 10.87% (10.45 ± 1.65 mmol/L, 10.74 ± 1.14 mmol/L vs. 12.05 ± 1.42 mmol/L, p < 0.05), respectively (B).
Change of Renal Histomorphology in Renal I/R Injury Rat
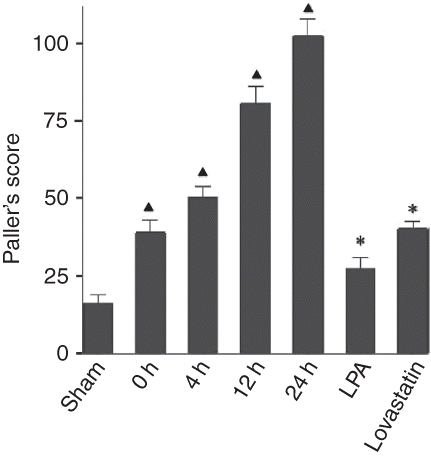
In sham-operated group, mild edema and lumen stenosis, but no other abnormality, were seen in epithelial cells of partial proximal convoluted tubule (B). In all I/R injury groups, with the extension of reperfusion, renal pathological alteration aggravated. Lumen surface of proximal convoluted tubules was uneven, brush border fell off and disappeared, and floccule and casts were seen in lumen (C and D). In 12 h and 24 h I/R groups, cellular edema was severe; necrosis and calcification of partial proximal convoluted tubules were visible (E and F). In 24 h I/R group, there was hyperplasia and congestion in partial kidney glomerulus and renal interstitium. In 4 h, 12 h, and 24 h I/R groups, there were a few round mononuclear cells in renal interstitium (D–F). Paller’s score was significantly increased in all I/R injury groups compared with sham-operated group (p < 0.01).
Figure 2. Renal histomorphology in renal ischemia/reperfusion (I/R) injury rat. H&E staining of renal tissue section from normal rat (A), sham-operated group (B), I/R 0 h (C), 4 h (D), 12 h (E), 24 h (F) groups, lysophosphatidic acid (LPA) treatment group (G), and lovastatin (H) treatment group. Bar represents 200 μm.
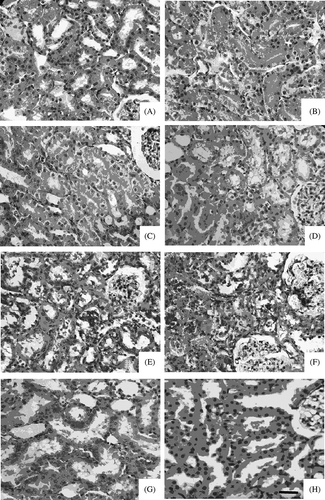
Compared with I/R injury groups, renal pathological alteration in LPA and lovastatin treatment groups was reduced. Lumen surface of proximal convoluted tubules was uneven, partial brush border fell off and disappeared, and there was mild edema in epithelial cells of proximal convoluted tubule and floccule in lumen. Pathological alteration was severer in lovastatin treatment group than in LPA treatment group (G and H). In lovastatin treatment group, there were casts in partial lumens and mild hyperemia round mononuclear cells in renal interstitium, but no obvious change in kidney glomerulus. Paller’s scores in LPA and lovastatin groups were significantly decreased compared with that in 4 h I/R group (p < 0.01), but still were higher than that in sham-operated group. At the same time, Paller’s score was significantly lower in LPA treatment group than in lovastatin treatment group ().
DISCUSSION
The damage of renal I/R injury is in part due to inflammatory response which leads to cell apoptosis and necrosis. Our study found that MCP-1, a chemotactic factor, was involved in the process. Our result showed that LPA and lovastatin might reduce the damages of renal histomorphology and function by downregulating the level of MCP-1.
LPA is a natural and biologically active phospholipid and also is an endogenous phospholipid growth factor which plays a role in anti-apoptosis. It conducts signal through three different G-protein-coupled receptors.Citation23 During renal I/R injury, LPA can prevent apoptosis and brush border injury of renal tubules and protect the kidney by inhibiting complement activity, interleukin induction, and neutrophilic granulocyte infiltration.Citation11 In rat models, the application of LPA exhibited a good effect on intestinal tract and skin injury.Citation24 Additionally, LPA also plays an important role in the occurrence and development of heart and cerebrovascular disease and kidney disease.Citation25,26
Our study found that the levels of MCP-1, Cr, and BUN and Paller’s score were much lower in LPA treatment group than in 4 h I/R group (p < 0.01), indicating that LPA can reduce renal damage and protect renal function. The likely mechanism is that during renal I/R injury, LPA can inhibit the body to produce a variety of chemotactic factors, which prevent mononuclear macrophage infiltration into renal tissue, and meanwhile LPA also plays a role in anti-apoptosis.
Statins are 3-hydroxy-3-3methy-1 glutaric aciduria acy-1 coenzyme A reductase inhibitors (HRI). Stains are classical and effective lipid-lowering drugs. Lovastatin belongs to statins. Previous studies about animal models with kidney damage have indicated that statins have direct protective effect on the kidney. Pisani et al.Citation19 used atorvastatin to treat rats with ischemic acute renal failure and found that atorvastatin could facilitate vascular endothelium to produce NO, which reduced renal vascular resistance and increased glomerular filtration rate. Zoja et al.Citation20 used simvastatin and lisinopril to treat rats with severe Heymann nephritis and found that compared with application of single drug, the two drugs combined could obviously improve renal function and reduce glomerular sclerosis, kidney tubule injury, and interstitial inflammation. These studies above cannot be explained by traditional lipid-lowering mechanism. In our study, lovastatin was used to prevent renal I/R injury, and results showed that compared with 4 h I/R group, levels of MCP-1, Cr, and BUN and Paller’s score decreased in lovastatin treatment group, indicating that lovastatin may reduce renal injury and protect renal function by downregulating MCP-1. Our results may explain the mechanism that how statins protect kidney. In renal I/R injury, HRI may inactivate NF-κB, which inhibits MCP-1 production,Citation7 and reduces mononuclear macrophage infiltration into renal tissue.
Figure 3. Paller’s scores in renal tissue section from sham-operated group, ischemia/reperfusion 0 h, 4 h, 12 h, and 24 h groups, lysophosphatidic acid (LPA) treatment group, and lovastatin treatment group. *p < 0.01 compared with sham-operated group. **p < 0.01, compared with ischemia/reperfusion 4 h group.
Renal I/R injury is a pathological change which is common in clinical practice. Our study showed that in 4 h, 12 h, and 24 h I/R groups, the level of MCP-1 increased, and there were a few round mononuclear cells in renal interstitial tissue, but not lots of inflammatory cells, indicating that the pathogenesis of renal I/R injury is complex, and is associated with a variety of factors such as ischemia, hypoxia, cytokine, and complement. At present, there are no effective methods to prevent renal I/R injury. Our study finds that LPA and lovastatin can reduce pathological alteration of rat renal tissue in renal I/R injury. Our study provides new methods and ideas for prevention and treatment of renal I/R injury.
ACKNOWLEDGMENTS
Our study is supported by Natural Science Foundation (grant no. 2007-110-20-919) and Scientific Research Project of Inner Mongolia Institution of Higher Education (no. NJ03130).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Agrawal M, Swartz R. Acute renal failure. Am Fam Physician. 2000;61:2077–2088.
- Chao IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621.
- Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Hemost. 2007;97:738–747.
- Jassem W, Fuggle SV, Rela M, Koo DD, Heaton ND. The role of mitochondria in ischemia/reperfusion injury. Transplantation. 2002;73:493–499.
- Daemen MA, de Vries B, Buurman WA. Apoptosis and inflammation in renal reperfusion injury. Transplantation. 2002;73:1693–1700.
- Duque N, Gomez-Guerrero C, Egido J. Interaction of IgA with Fc alpha receptors of human mesangial cells activates transcription factor nuclear factor-kappa B and induces expression and synthesis of monocyte chemoattractant protein-1, IL-8, and IFN-inducible protein 10. J Immunol. 1997;159:3474–3482.
- Kakio T, Matsumori A, Ono K, Ito H, Matsushima K, Sasayama S. Roles and relationship of macrophages and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the ischemic and reperfused rat heart. Lab Invest. 2000;80:1127–1136.
- Stroo I, Stokman G, Teske GJ, . Chemokine expression in renal ischemia/reperfusion injury is most profound during the reparative phase. Int Immunol. 2010;22:433–442.
- Furuichi K, Wada T, Iwata Y, . CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol. 2003;14:2503–2515.
- Furuichi K, Wada T, Iwata Y, . Gene therapy expressing amino-terminal truncated monocyte chemoattractant protein-1 prevents renal ischemia-reperfusion injury. J Am Soc Nephrol. 2003;14:1066–1071.
- de Vries B, Matthijsen RA, van Bijnen AA, Wolfs TG, Buurman WA. Lysophosphatidic acid prevents renal ischemia-reperfusion injury by inhibition of apoptosis and complement activation. Am J Pathol. 2003;163:47–56.
- Thurman JM, Lenderink AM, Royer PA, . C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol. 2007;178:1819–1828.
- Yuzbasioglu MF, Aykas A, Kurutas EB, Sahinkanat T. Protective effects of propofol against ischemia/reperfusion injury in rat kidneys. Ren Fail. 2010;32:578–583.
- Fouad AA, Qureshi HA, Al-Sultan Al, Yacoubi MT, Al-Melhim WN. Nephroprotective effect of telmisartan in rats with ischemia/reperfusion renal injury. Pharmacology. 2010;85:158–167.
- Horvath G, Racz B, Reglodi D, . Effects of PACAP on mitochondrial apoptotic pathways and cytokine expression in rats subjected to renal ischemia/reperfusion. J Mol Neurosci. 2010;42:411–418.
- Semedo P, Wang PM, Andreucci TH, . Mesenchymal stem cells ameliorate tissue damages triggered by renal ischemia and reperfusion injury. Transplant Proc. 2007;39:421–423.
- Zhuo W, Liao L, Xu T, Wu W, Yang S, Tan J. Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol Int. 2010;82(6):191–196; doi:10.1159/000319366.
- Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010;121:2211–2220.
- Pisani A, Uccello F, Cesaro A, . Effects of atorvastatin on ischemic acute renal failure in aging rats. G Ital Nefrol. 2002;19:534–539.
- Zoja C, Corna D, Rottoli D, . Effect of combining ACE inhibitor and statin in severe experimental nephropathy. Kindey Int. 2002;61:1635–1645.
- Lu JX, Ping JG, Yan CY. Establish of rat model of renal cold ischemia-reperfusion injury. Chin J Hemorheol. 2005;15:45–47.
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164.
- Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188–1196.
- Balazs L, Okolicany J, Ferrebee M, Tolley B, Tigyi G. Topical application of the phospholipid growth factor lysophosphatidic acid promotes wound healing in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;280:R466–R472.
- Inoue CN. LPA as a determinant of mesangial growth and apoptosis. Semin Nephrol. 2002;22:415–422.
- Chen X, Yang XY, Wang ND, . Serum lysophosphatidic acid concentrations measured by dot immunogold filtration assay in patients with acute myocardial infarction. Scand J Clin Lab Invest. 2003;63:497–503.