Abstract
During times of war or natural disasters, rhabdomyolysis leading to acute kidney injury (AKI) can assume epidemic proportions. Fasudil attenuates ischemia/reperfusion-induced AKI. We investigated the therapeutic effect of an early application of fasudil on AKI induced by rhabdomyolysis and explored the potential mechanisms. Male Wistar rats were randomly divided into a control group (saline, 7 mL/kg, i.m.), a Gly group (50% glycerol, 7 mL/kg, i.m.), and a fasudil group (50% glycerol, 7 mL/kg, i.m.; fasudil, 20 mg/kg bodyweight, i.p., three times every 24 h beginning 72 h before glycerol administration). Serum creatinine, blood urea nitrogen (BUN), and histopathological changes were used to demonstrate kidney function 24 h after the glycerol injection. Cell apoptosis and the expression of rho-associated protein kinase member (ROCK1), phosphatase and tensin homolog (PTEN), P-Akt, and caspase-8, -9, and -3 were measured. Serum creatinine and BUN levels increased significantly in Gly group compared with control group. Both levels decreased after fasudil treatment. The renal tubular damage score was significantly lower and cell apoptosis was significantly less in fasudil group compared with Gly group. The expression levels of ROCK1, PTEN, and caspase-8, -9, and -3 were upregulated significantly in Gly group, and their expression was reduced in the fasudil group. The P-Akt level was decreased in Gly group and upregulated significantly in fasudil group. Early application of fasudil reduced rhabdomyolysis-associated renal injury by inhibiting Rho kinase and thereby activating the PI-3K/Akt pathway, which decreased cell apoptosis via both the intrinsic and extrinsic apoptotic pathways.
INTRODUCTION
Rhabdomyolysis occurs when compression, exercise, fever, medication, or inflammation causes skeletal muscle destruction and disintegration, releasing creatine kinase, myoglobin, and other intracellular contents from myocytes into the circulation.Citation1 Acute kidney injury (AKI) is a severe complication of rhabdomyolysis, and the prognosis is poor once renal failure occurs. Approximately 10–40% of rhabdomyolysis cases result in induced AKI, accounting for 2–15% of all AKI cases.Citation2 The main pathophysiological mechanisms of rhabdomyolysis-induced myoglobinuric AKI are blood volume reduction, renal vasoconstriction, intraluminal cast formation, and direct myoglobin-induced cytotoxicity.Citation1,3 Mounting evidence indicates that cell apoptosis is also involved in the pathogenesis of rhabdomyolysis-induced AKI.Citation4 It is important to clarify the exact role of apoptosis in order to explore potential new therapies.
Rho-associated protein kinase (ROCK) is a serine/threonine protein kinase, initially identified as a Rho-binding protein. The ROCK family contains two members: ROCK1 and ROCK2. The interaction between ROCK and Rho regulates many biological activities, including smooth muscle contraction, cytoskeletal assembly, transcription factor activation, and cell cycle regulation.Citation5 Rho kinase also plays a critical role in apoptosisCitation6 by activating phosphatase and tensin homolog (PTEN), which then inhibits activation of the phosphatidylinositol 3-kinase/v-akt murine thymoma viral oncogene homolog (PI3-k/Akt) pathway and thereby promotes apoptosis.Citation7–9 Increasing evidence indicates that the inhibition of Rho kinase activity reduces apoptosis.Citation10,11
Fasudil, the first-reported Rho kinase-specific inhibitor,Citation12 has been shown to reduce brain and heart ischemia/reperfusion injury.Citation13–15 Rho kinase inhibitors have also been demonstrated to reduce ischemia/reperfusion renal injury in animal models.Citation16 However, the effect of Rho kinase inhibitors on myoglobinuric AKI is not known. In this study, we established a glycerol-induced AKI model, which is a classical rhabdomyolysis model of AKI in rodents,Citation1 and investigated the effect of fasudil on glycerol-induced myoglobinuric AKI in the rats.
MATERIALS AND METHODS
Materials
Major drugs and reagents
Rabbit polyclonal antibodies against caspase-8, -9, and -3; rabbit anti-Akt/P-AKt polyclonal antibody; mouse anti-ROCK1 monoclonal antibody; rabbit anti-PTEN polyclonal antibody; horseradish peroxidase-labeled goat anti-rabbit IgG; and rabbit anti-mouse IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-β-actin monoclonal antibody was obtained from Sigma (St. Louis, MO, USA). Injectable fasudil hydrochloride was from Tianjin Pharmaceutical Co. (Tianjin, PR China). A transferase biotin-dUTP nick end-labeling (TUNEL) staining kit (DeadEnd™ Colorimetric TUNEL System) was purchased from Promega (Madison, WI, USA).
Animals
Wistar male rats (200–250 g) were purchased from the Experimental Animal Center of Military Medical Sciences (Beijing, PR China). The rats were maintained in an air-conditioned room with a 12 h light–dark cycle, a temperature of 19–29°C, and a relative humidity of 40–70%. This study protocol was approved by the ethics committee of PLA General Hospital.
Study Design
Animal groups and establishment of the animal model
Thirty male Wistar rats were randomly divided into a control group (control, n = 10), a Gly group (Gly, n = 10), and a fasudil group (fasudil, n = 10). The Gly group rats were deprived of water for 24 h and then administered half of the dose of glycerol (50% v/v in sterile saline; 7 mL/kg bodyweight) in each hind limb muscle, under light sedation with pentobarbital. The fasudil group rats received fasudil i.p. (20 mg/kg bodyweight) one time every 24 h beginning 72 h before glycerol administration. The control group rats were deprived of water for 24 h and then administrated saline (0.9% v/v in sterile water; 7 mL/kg bodyweight). At 24 h after injection, all of the rats were killed, and kidney morphology and renal function were determined.
Renal Function Studies
Serum urea and creatinine were assessed with a Hitachi 7150 automatic biochemical analyzer.
Histological Analysis
Kidney tissue was fixed in 10% formalin for 24 h for routine dehydration and paraffin embedding. Sections were cut at 2 mm and stained with periodic acid-Schiff. The slides were coded, and a pathologist (JC), who was unaware of the group assignments of the tissues, performed a semiquantitative analysis of the kidney sections by Paller’s method,Citation17 scoring 100 cortical tubules from at least 10 different areas from each kidney. Higher scores represented more severe damage, with a maximum score of 10 points per tubule. Points were given for the presence and extent of tubular epithelial cell flattening (1 point), brush border loss (1 point), cell membrane bleb formation (1 or 2 points), interstitial edema (1 point), cytoplasmic vacuolization (1 point), cell necrosis (1 or 2 points), and tubular lumen obstruction (1 or 2 points).
In Situ Terminal Deoxynucleotidyl TUNEL Assay
A TUNEL assay was used to detect apoptotic DNA fragmentation. Kidney tissue was fixed in 10% formalin overnight, dehydrated, embedded in paraffin, cut at 2 mm thickness sections, and placed on a numbered polylysine-coated glass slide. TUNEL staining (brown) was performed with an in situ cell death detection kit (Promega) according to the manufacturer’s instructions. Nuclei were stained with hematoxylin to observe the nature of the TUNEL-positive cells. TUNEL-positive nuclei were expressed as a percentage of total nuclei [4′,6-diamidino-2-phenylindole (DAPI) positive] per field. Six to eight fields per section and two to three sections per kidney were examined in each experiment.Citation18
Table 1. Serum creatinine (Scr) and blood urea nitrogen (BUN) concentrations.
Western Analysis
Frozen kidney tissue was homogenized for radioimmunoprecipitation. The homogenates were clarified by centrifugation at 12,000 × g for 10 min at 4°C, and the supernatants were collected. Supernatant samples (70 μg) were subjected to 15% polyacrylamide gel electrophoresis and transferred to cellulose acetate membranes. The membranes were blocked with 1× casein solution for about 4 h and then incubated with rabbit polyclonal anti-caspase-8, -9, and -3; rabbit polyclonal anti-AKt/P-AKt; rabbit polyclonal anti-PTEN; and mouse monoclonal anti-ROCK1 antibodies overnight at 4°C. The blots were washed with Tris-Buffered Saline Tween-20 (TBST) buffer and subsequently incubated with goat anti-rabbit or rabbit anti-mouse IgG. After washing with TBST, the blots were developed with enhanced chemiluminescence reagents. A mouse monoclonal anti-β-actin antibody was used as a control for each sample.
Western blots were analyzed by the free image analyzing software Image J 1.42 (downloaded from http://rsbweb.nih.gov/ij/download.html) and its certain plugins.
Statistical Analysis
Measurement data are presented as means ± standard deviation. Statistical analyses were performed using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA). The significance of experimental differences between groups was evaluated using Student’s t-test. Nonparametric data were analyzed by the rank-sum test. A p-value of p < 0.05 indicated a significant difference.
RESULTS
Renal Function and Histopathology
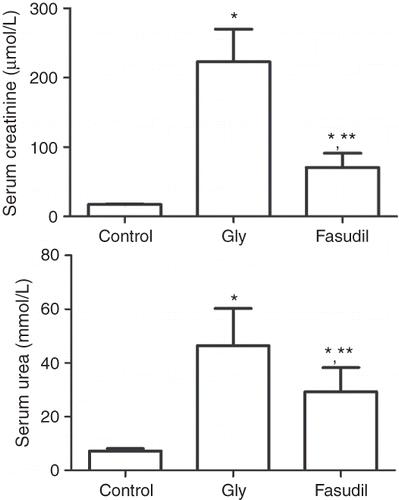
Glycerol administration resulted in significant increases in the serum creatinine and blood urea nitrogen (BUN) levels in the Gly group compared with the control group (). Inhibition of Rho kinase activity by fasudil resulted in significantly improved renal function, with significantly lower serum creatinine and BUN levels compared with the levels in the Gly group ().
Figure 1. Serum creatinine (A) and blood urea nitrogen (B) concentrations.
Note:*p < 0.01 versus control group, **p < 0.01 versus Gly-model group, n = 10.
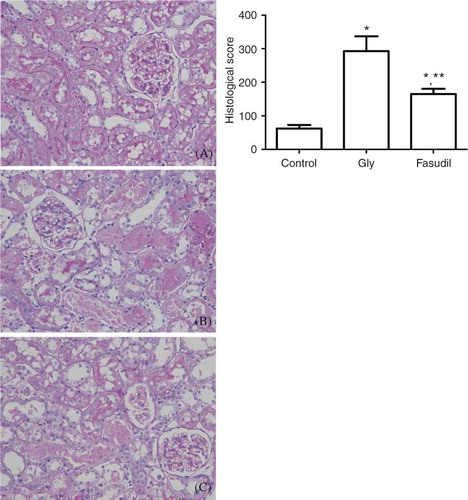
The control group showed no histopathological changes under light microscopic examination. In contrast, the basic histological abnormalities in the Gly group and fasudil group were tubular necrosis and cast formation. Necrosis was most severe in the cortical segments of the proximal tubules, with less-extensive changes in the medullar segment of the kidney. The tubulointerstitial histological damage score was significantly lower in the fasudil group than in the Gly group ().
Figure 2 Periodic acid-Schiff-stained sections of rat kidneys. (A) Normal kidney section. (B) Kidney section of glycerol-treated rat showing tubular necrosis and cast formation. (C) Kidney section of a fasudil (20 mg/kg) and glycerol-treated rat showing morphological damage significantly improved.
Note: *p < 0.05 versus control group, **p < 0.05 versus Gly group, n = 10.
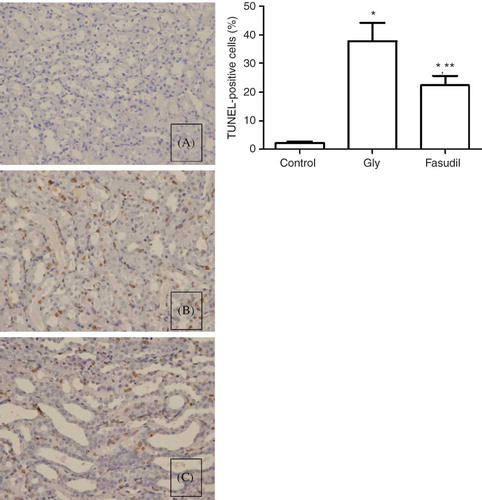
Severity of Apoptosis
In the TUNEL assay, the nuclei of TUNEL-positive cells were stained brown, indicating apoptotic cells. Few apoptotic cells were seen in the control group (0.21 ± 0.082), whereas the Gly group displayed markedly more TUNEL-positive cells (37.85 ± 6.32), which were observed mainly in the corticomedullary junction and medullary area, with occasional occurrence in the cortex. Some TUNEL-positive cells were detached from the tubular basement membrane in the lumen. Fasudil treatment decreased the number of TUNEL-positive cells, and fewer apoptotic cells were observed in the fasudil group compared with the Gly group (22.35 ± 3.39 vs. 37.85 ± 6.32, respectively, p < 0.05) ().
Figure 3. Determination of tubular cell apoptosis in situ terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) assay. (A) Few apoptotic cells were seen in the control group. (B) Gly group displayed markedly more TUNEL-positive cells, mainly in the corticomedullary junction and medullary area. (C) Fasudil treatment decreased the number of TUNEL-positive cells, and fewer apoptotic cells were observed in the fasudil group.
Note: **p < 0.05 versus control group, **p < 0.05 versus Gly group, n = 10.
ROCK1, PTEN, and Akt/P-Akt Protein Expression
ROCK1 protein expression was increased significantly in the Gly group compared with the control group. With fasudil treatment, ROCK1 protein expression was significantly downregulated compared with the levels in the Gly group. Similarly, the PTEN expression level was significantly higher in the Gly group than in the control group, and PTEN expression was significantly reduced by fasudil treatment. The expression of total Akt protein in each group was at the same level. While the level of phosphorylated Akt was significantly lower in the Gly group compared with the control group, the phosphorylated Akt level was significantly upregulated in the fasudil group compared with the Gly group ().
Figure 4. ROCK1, PTEN, and Akt/P-Akt protein expressions. (A) Densitometric quantifications of band intensities from western blot for ROCK1, PTEN, P-Akt, Akt/β-actin. ROCK1 protein expression was increased significantly in the Gly group compared with the control group. With fasudil treatment, ROCK1 protein expression was significantly downregulated compared with the levels in the Gly group. The expression of total Akt protein in each group was at the same level, while the level of phosphorylated Akt was significantly lower in the Gly group compared with the control group, and the phosphorylated Akt level was significantly upregulated in the fasudil group compared with the Gly group. (B) Representative tissue ROCK1, PTEN, and Akt/P-Akt protein expression.
Note: *p < 0.05 versus control group, **p < 0.05 versus Gly group, n = 10.
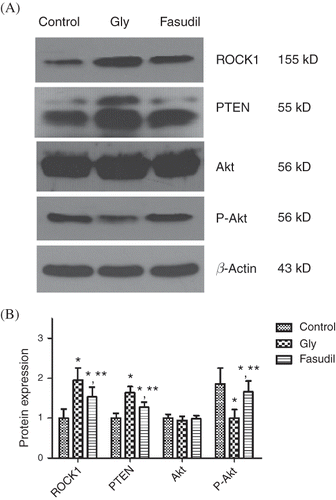
Figure 5. Caspase-8, 9, 3 protein expressions. (A) Densitometric quantifications of band intensities from western blot for caspase-8, 9, 3/β-actin. The levels of caspase-8, -9, and -3 were significantly higher in the Gly group than in the control group. Fasudil treatment significantly reduced the levels of caspase-8, -9, and -3 compared with the levels in the Gly group. (B) Representative tissue caspase-8, 9, 3 protein expression.
Note: *p < 0.05 versus control group, **p < 0.05 versus Gly group, n = 10.
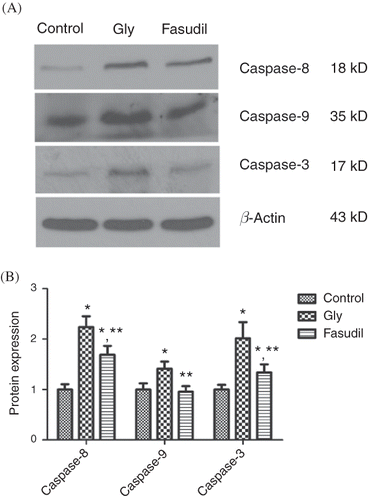
Caspase-8, -9, and -3 Protein Expressions
The levels of caspase-8, -9, and -3 were significantly higher in the Gly group than in the control group. Fasudil treatment significantly reduced the levels of caspase-8, -9, and -3 compared with the levels in the Gly group ().
DISCUSSION
Rehydration, maintenance of fluid and electrolyte balances, supportive treatment, and blood purification are currently the main therapeutic approaches for rhabdomyolysis. Unfortunately, some patients still progress to AKI.Citation19 Oxidative stress, inflammation, and renal tubular epithelial cell necrosis play important roles in rhabdomyolysis-induced AKI, and the application of l-carnitine, carvedilol,Citation20,21 and hepatocyte growth factor Citation22,23 can attenuate rhabdomyolysis-induced AKI. However, these agents are not yet used clinically. Fasudil is a specific inhibitor of Rho kinase, and its application can reduce brain and heart ischemia/reperfusion injury. Rho kinase inhibitors have also been shown to reduce ischemia/reperfusion renal injury in animal models.Citation16 Here, we investigated the therapeutic effect of fasudil treatment on AKI induced by rhabdomyolysis.
The most widely used rhabdomyolysis model of AKI in rodents is glycerol-induced AKI,Citation1,24–26 which involves renal vasoconstriction, hypoperfusion, direct myoglobin-induced cytotoxicity, and the release of activated factors from the kidneys, as in rhabdomyolysis-induced AKI. In our study, ahead of schedule we tested the serum creatinine-phosphokinase (CPK) concentrations in each group at 6 and 12 h. We found the serum CPK concentrations in the Gly group and the fasudil group were significantly increased at 6 h compared with the control group, and a trend was even more obvious at 12 h. However, there was no significant difference between these two groups. So fasudil played a minimum role in the serum CPK concentrations in the Gly group and the fasudil group. At 24 h, the Gly group exhibited significantly increased serum creatinine and BUN levels and significant morphological changes, including vacuolar degeneration, brush border loss, cast formation, severe tubular necrosis, and tubulorrhexis, of many proximal tubules. With fasudil treatment, the serum creatinine and BUN levels, renal tubular epithelial cell degeneration and necrosis, and tubule necrosis scores were all significantly reduced. These results indicate that fasudil treatment significantly improved the renal function and reduced the morphological damage induced by rhabdomyolysis, while playing a minimum role in rhabdomyolysis.
Renal tubular epithelial cell apoptosis is important in the pathogenesis of rhabdomyolysis-induced AKI.Citation4 In our study, TUNEL staining revealed numerous apoptotic cells in the kidneys of Gly group rats, whereas the number of apoptotic cells was significantly decreased in the rats treated with fasudil, suggesting that treatment with a Rho kinase inhibitor may significantly reduce apoptosis.
The results of the present study demonstrate the importance of Rho kinase (ROCK1 and ROCK2) in the regulation of apoptosis.Citation6 Rho kinase activates PTEN, thereby inhibiting the PI3K/Akt pathway and increasing apoptosis.Citation7–9 We hypothesized that the specific Rho kinase inhibitor fasudil would reduce apoptosis by preventing PTEN activation. Both ROCK1 and ROCK2 are ubiquitously expressed in humans, rats, and mice. ROCK2 is more prominent in brain and skeletal muscle, whereas ROCK1 is predominant in liver, testes, and kidney.Citation6 In this study, we simultaneously measured the protein expression level of ROCK1 and of PTEN, a specific Rho kinase substrate.Citation27 Both ROCK1 and PTEN were upregulated in rhabdomyolysis-induced AKI animals, and fasudil treatment significantly reduced ROCK1, PTEN expression, and apoptosis. Thus, Rho kinase may be involved in the increased cell apoptosis associated with rhabdomyolysis-induced AKI.
The PI3K/Akt pathway is an important cell survival signaling pathway,Citation28 and as a survival factor, phosphorylated Akt can result in reduced cell apoptosis.Citation7,29,30 Conversely, inhibition of the PI3K/Akt pathway can lead to kidney cell apoptosis, and this approach has been used to treat kidney tumors.Citation31 A previous study has demonstrated that renal cell apoptosis and cisplatin-induced AKI are significantly serious by PI3K knockout.Citation32 It is reported that Rho kinase can activate PTEN to inhibit activation of the PI3-k/Akt pathway and thereby promotes apoptosis.Citation7–9 In the present study, the expression of total Akt protein in each group was at the same level, while P-Akt expression was decreased in the Gly group following intramuscular injection of glycerol. When Rho kinase activity was inhibited by fasudil treatment, P-Akt expression was significantly upregulated, showing that inhibition of Rho kinase can activate PI3K/Akt pathway.
The caspases are critical effectors of apoptosis and are involved in both the mitochondrial (intrinsic) and the death receptor (extrinsic) apoptotic pathways. In the mitochondrial pathway, inflammation, oxidative stress, and other factors’ stimuli activate caspases through the activation of the Bcl-2 family proteins, such as Bid, Bad, Bim, and Nix, then activate Bax and Bak, leading to the release of cytochrome c from the mitochondria, and cytosolic cytochrome c facilitates caspase-9 activation.Citation33,34 In the death receptor pathway, the binding of extracellular ligands such as tumor necrosis factor-α and Fas to their cell-surface receptors leads to caspase-8 activation. Caspase-8 and -9 cleave caspase-3 to initiate apoptosis. As the primary executor of caspase-dependent apoptosis, caspase-3 cleaves many structural and regulatory proteins in the cell membrane, cytoplasm, and nucleus causing a series of morphological and molecular changes that result in cell death.Citation35 In our study, the activated caspase-8, -9, and -3 levels were significantly increased in rhabdomyolysis-induced AKI and were significantly decreased by fasudil. It is reported that PI3K-dependent survival kinases directly phosphorylate and inactivate the proapoptotic Bcl-2 protein Bad,Citation36 and activated Akt can phosphorylate and inactivate members of Fas-dependent apoptosis.Citation7 So we speculate that the downregulation of PTEN and upregulation of P-Akt by fasudil could affect caspase-8, -9, and -3 through its downregulation of the downstream apoptotic factors Bcl-2 protein Bad and Fas. Thus, fasudil may reduce apoptosis by affecting both apoptosis regulation and execution.
Apoptosis and necrosis are two types of cell deaths that are both regulated by the caspase family proteins. Among the total, capase-8 not only can affect apoptosis but also have an effect on necrosis.Citation37,38 Renal tubular epithelial cell apoptosis and necrosis could be found in Gly group with TUNEL or periodic acid-Schiff (PAS) stain, while they are significantly decreased in fasudil group; this phenomenon might be a result of part of the caspase family proteins which could be regulated via Rho/PI-3K/Akt pathway by fasudil.
In summary, early application of the Rho kinase inhibitor fasudil reduced rhabdomyolysis-induced AKI. Fasudil inhibited Rho kinase and thereby activated the PI-3K/Akt pathway, which decreased cell apoptosis via both the intrinsic mitochondrial and extrinsic death receptor apoptotic pathways. This represents a potential mechanism of the beneficial effects of fasudil on rhabdomyolysis-induced AKI.
ACKNOWLEDGMENTS
This work was supported by the Fund of Chinese PLA Eleventh Five-Year Plan for Medical Sciences (nos. 08Z034, 06Q065) and a grant from the National Basic Research Program of China (nos. 2007CB507400, 2011CB944000).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49:314–326.
- Beetham R. Biochemical investigation of suspected rhabdomyolysis. Ann Clin Biochem. 2000;37(Pt. 5):581–587.
- Abassi ZA, Hoffman A, Better OS. Acute renal failure complicating muscle crush injury. Semin Nephrol. 1998;18:558–565.
- Homsi E, Janino P, de Faria JB. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006;69:1385–1392.
- Takuwa Y. Rho-rho kinase pathway. Nippon Rinsho. 2004;62:43–48.
- Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz). 2007;55:61–75.
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390.
- Chang J, Xie M, Shah VR, . Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci USA. 2006;103:14495–14500.
- Li Z, Dong X, Wang Z, . Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404.
- Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun. 2003;306:244–249.
- Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1168–L1178.
- Uehata M, Ishizaki T, Satoh H, . Calcium sensitization of smooth muscle mediated by a rho-associated protein kinase in hypertension. Nature. 1997;389:990–994.
- Rikitake Y, Kim HH, Huang Z, . Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257.
- Satoh S, Utsunomiya T, Tsurui K, . Pharmacological profile of hydroxy fasudil as a selective rho kinase inhibitor on ischemic brain damage. Life Sci. 2001;69:1441–1453.
- Bao W, Hu E, Tao L, . Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res. 2004;61:548–558.
- Teraishi K, Kurata H, Nakajima A, Takaoka M, Matsumura Y. Preventive effect of Y-27632, a selective Rho-kinase inhibitor, on ischemia/reperfusion-induced acute renal failure in rats. Eur J Pharmacol. 2004;505:205–211.
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984;74:1156–1164.
- Zhu T, Au-Yeung KK, Siow YL, Wang G, O K. Cyclosporine a protects against apoptosis in ischemic/reperfused rat kidneys. Clin Exp Pharmacol Physiol. 2002;29:852–854.
- Heyne N, Guthoff M, Weisel KC. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:1412–1413.
- Ustundag S, Sen S, Yalcin O, Ciftci S, Demirkan B, Ture M. l-Carnitine ameliorates glycerol-induced myoglobinuric acute renal failure in rats. Ren Fail. 2009;31:124–133.
- Singh D, Chander V, Chopra K. Carvedilol, an antihypertensive drug with antioxidant properties, protects against glycerol-induced acute renal failure. Am J Nephrol. 2003;23:415–421.
- Homsi E, Janino P, Amano M, Saraiva Camara NO. Endogenous hepatocyte growth factor attenuates inflammatory response in glycerol-induced acute kidney injury. Am J Nephrol. 2009;29:283–291.
- Nagano T, Mori-Kudo I, Tsuchida A, Kawamura T, Taiji M, Noguchi H. Ameliorative effect of hepatocyte growth factor on glycerol-induced acute renal failure with acute tubular necrosis. Nephron. 2002;91:730–738.
- Hsu CH, Kurtz TW, Waldinger TP. Cardiac output and renal blood flow in glycerol-induced acute renal failure in the rat. Circ Res. 1977;40:178–182.
- Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: Role of iron in nephrotoxicity. Am J Physiol. 1988;255:F539–F544.
- Homsi E, Oliveira Dias EP, Garcia WE, Gontijo JA, Figueiredo JF. Effects of nifedipine and platelet activating factor antagonist (BN 52021) in glycerol-induced acute renal failure in rats. Ren Fail. 1996;18:883–892.
- Papakonstanti EA, Ridley AJ, Vanhaesebroeck B. The p110delta isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J. 2007;26:3050–3061.
- Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624.
- Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res. 2004;94:7–16.
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71.
- Sourbier C, Lindner V, Lang H, . The phosphoinositide 3-kinase/Akt pathway: A new target in human renal cell carcinoma therapy. Cancer Res. 2006;66:5130–5142.
- Kuwana H, Terada Y, Kobayashi T, . The phosphoinositide-3 kinase gamma-Akt pathway mediates renal tubular injury in cisplatin nephrotoxicity. Kidney Int. 2008;73:430–445.
- Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219.
- Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571.
- Padanilam BJ. Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:F608–F627.
- Datta SR, Dudek H, Tao X, . Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241.
- Peter ME. Programmed cell death: Apoptosis meets necrosis. Nature. 2011;471:310–312.
- Hetz CA, Hunn M, Rojas P, Torres V, Leyton L, Quest AF. Caspase-dependent initiation of apoptosis and necrosis by the Fas receptor in lymphoid cells: Onset of necrosis is associated with delayed ceramide increase. J Cell Sci. 2002;115:4671–4683.