Abstract
Background and aims: Hepatitis C virus (HCV) is now recognized as one of the major causes of chronic liver disease. It is also one of the most common complications in maintenance hemodialysis (HD) patients. Tumor necrosis factor (TNF)-α promoter polymorphisms are observed to modulate TNF-α levels and thought to have an effect on susceptibility to HCV infection and the virus clearance, but the results are inconsistent. In this study, a systematic review and meta-analysis of the published data was performed to evaluate the relationship between the TNF-α-238, -308 polymorphisms and HCV infection. Methods: A total of 15 studies published were analyzed, which were indexed from PubMed, Embase, and CNKI databases (up to December 2010). All the data were analyzed using RevMan 4.2 software. Odds ratios (OR) and confidence intervals (95% CI) were calculated by fixed or random-effects models. Heterogeneity and publication bias across the studies were also explored. Results: The data showed no significant association between TNF-α-308, -238 gene polymorphisms and the susceptibility to HCV infection in the global group (p = 0.28, p = 0.38, respectively) and the sub-groups (European, American, African, and Asian). Besides, the distributions of TNF-α-308, -238 A/G alleles were also not significantly different between the persistent infection group and the spontaneous clearance group (p = 0.64, p = 0.75, respectively). Conclusion: TNF-α-238, -308 gene polymorphisms might have no effect on susceptibility to HCV infection and the virus clearance. The findings of this meta-analysis have implications in the optimal prevention of HCV in HD patients and in the guidance of future research.
INTRODUCTION
Hepatitis C virus (HCV) is now recognized as one of the major causes of chronic liver disease, and approximately 170 million are infected worldwide.Citation1,2 As we know, hemodialysis (HD) is an effective means for the treatment of end-stage renal disease and prolongs lives of patients with uremia, which has been widely used in clinic. However, because of long-term blood transfusion, the low immune ability of the body, as well as cross-iatrogenic infection, HD patients are highly vulnerable to viral hepatitis infection.Citation3 In recent years, several studies suggest HCV infection rate has been up to the first in HD patients, as high as 34.3–42.6%.Citation4,5 HCV infection in HD patients not only affects the quality of lives, but also increases the mortality rate and complications, and declines the long-term survival rate of renal transplantation.Citation6
There are various kinds of consequences after infection: some will remain asymptomatic, however, other patients will have severe diseases, which may result in necrosis of liver cells, liver fibrosis, dysfunction, cirrhosis, hepatocellular carcinoma, and finally lead to death.Citation7–9 There are a number of factors, such as age, sex, alcohol consumption, viral genotype, individual’s genotype, routes of infection, duration of infection, and complication of other diseases (such as uremia), that had been conceived a variable significance in determining the natural history and the consequence of HCV infection, including spontaneous clearance of the virus and all the different consequences caused by persistent infection.
The mechanisms with respect to the infection, persistence, and pathogenesis of HCV are still not clear. So far, analyses of the outcome of the infection have mainly focused on genetics, virological, and immunological factors.Citation10 Previous studies have indicated that the host variation of cytokine levels plays an important role in inflammatory and immune responses after the infection of HCV. The production of inappropriate levels of tumor necrosis factor (TNF)-α, TNF-β, interleukin-10, interleukin-12, and some other kinds of cytokines are reported to contribute to viral persistence, to influence the function of liver, and to affect response to antiviral therapy.Citation11–16 Among these cytokines, TNF-α is a multifunctional immune modulator encoded by the major histocompatibility complex class III. It can recruit and activate macrophages, NK cells, and T cells for their effectors’ functions including the production of immune regulatory and antiviral cytokines, which promote the inflammatory response of liver cells and mediate liver cell injury, or lead to the persistence of chronic inflammatory liver.
It has been shown that cytokine genes are polymorphic at specific sites, and it has been suggested that some of these mutations located within coding or regulatory regions may affect the overall production of the associated cytokine and the host immune response in several infectious diseases. Associations between polymorphisms in cytokine genes and inflammation, autoimmune syndromes, allograft rejection, and infectious diseases have been reported.Citation17,18 Several polymorphic sites within the promoter region of the TNF-α gene have been described; the combination of two main single nucleotide polymorphisms at positions -238 and -308 from the transcription start site produces three different haplotypes (GA or AA, and GG), which are associated with either higher or lower production of TNF-α expression.Citation19,20
Current studies are more concerned with the relationship between polymorphisms of TNF-α promoter position at -308, -238 and the outcome of HCV infection. However, the results of previous studies were inconsistent. The complexity of HCV infection and the quality of the small sample associated with studies may influence the accuracy of the results, so we carried out a meta-analysis on TNF-α polymorphism and HCV infection in order to improve the statistical power and get a more definitive conclusion about the relationship between the polymorphisms at TNF-α-308, -238 and the susceptibility and the outcome of HCV infection, and help to provide a reliable basis to formulate the prevention and control plans, and also to give a clear direction for future researches.
MATERIALS AND METHODS
Publication Search
Four electronic databases (PubMed, Embase, Wan Fang-data, and CNKI Database) were searched (last search was updated on 15 December 2010, using the key words and search terms: “TNF-α,” “polymorphism,” and “HCV”) to identify potentially concerned published papers in English and Chinese. All eligible studies were carefully extracted from all relevant publications, and their bibliographies were checked for other relevant publications. Only published studies with full text articles were included. The following data showed in and were collected from each study: surname of first author, publication date, country of origin, total number of cases and controls, and numbers of cases and controls with TNF-α-308, -238 genotypes, respectively. Different ethnicity descents were categorized as European, American, African, and Asian.
Table 1. Characteristics of included studies investigated the relationship between TNF-α polymorphisms and HCV infection.
Inclusion Criteria
The studies included in the meta-analysis had to meet the following criteria: (1) evaluated the TNF-α polymorphism and HCV infection, (2) were case–control studies, (3) an original article, (4) the article must provide the number of genotype frequency, and (5) a human study.
Exclusion Criteria
The studies excluded in the meta-analysis met all the following criteria: (1) the description for gene frequency distribution of polymorphism was not given, (2) the study without healthy controls, (3) letters and review articles, (4) studies not specifying sample origins, and (5) a subset of a published article by the same authors. We did not define any minimum number of patients to include a study in our meta-analysis.
Statistical Analysis
Owing to the heterogeneity between the studies, the odds ratio (OR) with 95% CI was used to estimate the strength of association between the TNF-α-308, -238 polymorphisms and the outcome of HCV infection for each study. According to the results of heterogeneity tests, we used random effects model or fixed-effect model to study. The reasons of heterogeneity may summarize as the differences of races, ratio of sex, limited number of patients, and so on. In order to study racial factors on the impact of this study, we performed the subgroup study according to the racial population. Because the literatures had not yet given the data of genotypes about sex and age, we did not carry out these kinds of subgroup analyses. Since publication bias is of concern for our meta-analysis, publication bias was investigated using a funnel plot, and an asymmetric plot suggested possible publication bias. Analyses were performed using the software Review Manager 4.2 (The Cochrane Collaboration, Oxford, UK, 2003). p-Values were at 0.05 level of statistical significance with the two-sided test.
RESULTS
Study Characteristics
Studies relevant to the searching words were retrieved originally. There were 15 eligible studiesCitation21–30,32–36 as a result of the search and screening carried out on the basis of our eligibility criteria, which had assessed the association between TNF-α-308, -238 genotypes and risk of HCV infection using human genomic DNA samples. The characteristics of selected studies are summarized in and . Among the 15 eligible studies, 14 were published in English and 1 was in Chinese. The total number of cases and controls in these studies were 1650 and 1386, and the number of patients with persistent infection or spontaneous clearance was 793 and 299, respectively. Healthy controls were mainly healthy populations. There were three subjects of Asians,Citation22,29,34 nine subjects of Europeans,Citation21,23,25–28,32,33,35 three subjects of Americans,Citation36,24,30 and one subject of Africans.Citation24
Table 2. Characteristics of included studies investigated the relationship between TNF-α polymorphisms and spontaneous clearance of HCV infection.
Overall Effects for Alleles
HCV patients and healthy controls
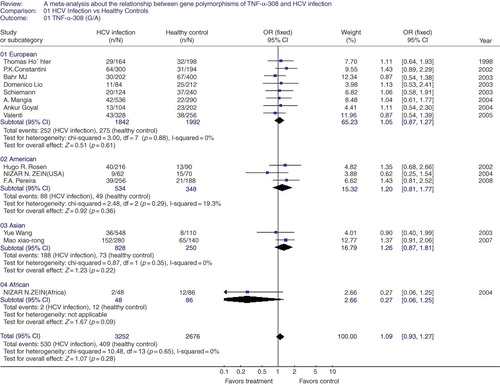
TNF-α-308 G/A polymorphism in HCV infection. In this part of meta-analysis, there are 1650 HCV patients and 1386 healthy controls. There was no heterogeneity between the involved studies, so a fixed-effect model was used. We found there is no significant association between TNF-α-308 G/A polymorphism and HCV infection in the global people (OR = 1.09, 95% CI = 0.93–1.27, p = 0.28, ). In order to look for ethnic effect, the subgroup analysis was also carried out. Still no evidence showed susceptibility to the HCV infection through the subgroup analysis under a fixed-effect model (group of European: OR = 1.05, 95% = CI 0.87–1.27, p = 0.61; group of American: OR = 1.20, 95% CI = 0.81–1.77, p = 0.36; group of Asian: OR = 1.26, 95% CI = 0.87–1.81, p = 0.22; group of African: OR = 0.27, 95% CI = 0.06–1.25, p = 0.09, ). Besides, the funnel plot was asymmetrical, suggesting publication bias among the studies.
Figure 1. A meta-analysis about the relationship between gene polymorphisms of tumor necrosis factor (TNF-α-308(G/A) and hepatitis C virus (HCV) infection in global group and subgroups. The pooled OR and 95% CI were generated using a fixed-effect model. Studies are ordered by publication year in each subgroup.
Note: OR, odds rate; CI, confidence interval; df, degrees of freedom.
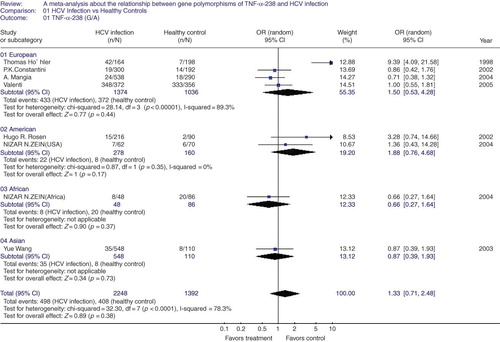
TNF-α-238 G/A polymorphism in HCV infection. We also performed the heterogeneity test of TNF-α-238 G/A polymorphism in HCV infection, which showed statistical significance (p < 0.0001), indicating the heterogeneity in the eight articles included; hence a random effect was used in the global study. In this meta-analysis with 1125 cases and 696 controls, we also found no association between TNF-α-238 G/A polymorphism and HCV infection in the global population (OR = 1.33, 95% CI = 0.71–2.48, p = 0.38, ). In order to study the impact of racial factor, we conducted a subgroup study. There was appearance of heterogeneity between the four articles of European group (p < 0.00001), so a random-effect model was only used to pool the results in this subgroup. The results demonstrated that there was no significant difference of HCV infection rates between the TNF-α-238 G/A polymorphism and HCV infection with the racial factors (group of European: OR = 1.50, 95% CI = 0.53–4.28, p = 0.44; group of American: OR = 1.88, 95% CI = 0.76–4.68, p = 0.17; group of African: OR = 0.66, 95% CI = 0.27–1.64, p = 0.37; group of Asian: OR = 0.87, 95% CI = 0.39–1.93, p = 0.73, ).
Figure 2. A meta-analysis about the relationship between gene polymorphisms of tumor necrosis factor (TNF-α-308(G/A) and hepatitis C virus (HCV) infection in global group and subgroups. The pooled OR and 95% CI were generated using a random-effect model. Studies are ordered by publication year in each sub-group.
Note: OR, odds rate; CI, confidence interval; df, degrees of freedom.
Persistent infection and resolved infection
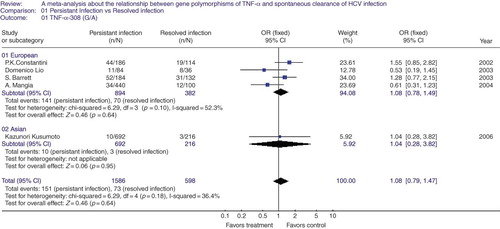
TNF-α-308 G/A polymorphism in persistent infection and resolved infection. Five studies were included in this part of analysis. According to the results of heterogeneity test between the involved studies, a fixed-effect model was used to poll the experimental data. There was still no evidence that the allele studied was associated with resolved infection after HCV infection (OR = 1.08, 95% CI = 0.79–1.47, p = 0.64, ). The subgroup analysis in population of European and Asian descent was also performed. Expectably, no effect of TNF-α-308 G/A alleles on spontaneous clearance was seen in the above subgroups (European group: OR = 1.08, 95% CI = 0.78–1.49, p = 0.64; Asian group: OR = 1.04, 95% CI = 0.28–3.82, p = 0.95, ). The funnel plot was symmetrical, suggesting there is no publication bias.
Figure 3. A meta-analysis about the relationship between gene polymorphisms of tumor necrosis factor (TNF)-α-308(G/A) and spontaneous clearance of hepatitis C virus (HCV) in global group and subgroups. The pooled OR and 95% CI were generated using a fixed-effect model. Studies are ordered by publication year in each sub-group.
Note: OR, odds rate; CI, confidence interval; df, degrees of freedom.
TNF-α-238 G/A polymorphism in persistent infection and resolved infection. In this part of meta-analysis, the number of persistent infection and resolved infection about -238 G/A was 659 and 215, respectively. The heterogeneity test showed no statistical significance between the three studies, so a fixed-effect model was used. As shown in , the OR was 1.11, 95% CI = 0.58–2.15, p = 0.75, in TNF-α-238 G/A alleles, which means that no evidence indicated that such alleles were associated with the natural clearance of the HCV. And the number of studies is too limited to perform a subgroup analysis. The funnel plot was not asymmetrical, meaning no public bias.
Figure 4. A meta-analysis about the relationship between gene polymorphisms of tumor necrosis factor (TNF)-α-238(G/A) and spontaneous clearance of hepatitis C virus (HCV) in global group. The pooled OR and 95% CI were generated using a fixed-effect model. Studies are ordered by publication year.
Note: OR, odds rate; CI, confidence interval; df, degrees of freedom.

DISCUSSION
HCV is one of the most important causes of chronic liver disease worldwide, and it is also a common risk factor of HD patients. The declined immune function in uremic patients undergoing HD, especially the cellular immunity, together with defection of neutrophil function and complement activity, weaken the ability of resisting pathogenic micro-organisms and pathogenic virus in HD patients.Citation3,37,38 According to recent studies, HBV infection rate is 6–7.8% and HCV 20–30% in HD patientsCitation39,40 and HD does not increase the prevalence of hepatitis B, but significantly increases hepatitis C infection rates.Citation41,42
The natural outcome of HCV infection varies dramatically among individuals. HCV infection is self-limited in a fortunate minority, while the majority of subjects develop persistent chronic infection. As it was shown in the epidemiological survey, only 15% of the acute HCV infection can completely remove the virus, the remaining 85% of those infected were formed of different types of chronic infection.Citation11 Its outcome is determined by various kinds of host factors and viral factors. The former includes race, age, genes, the combination of other chronic diseases, the appropriateness of treatment, and so on. Although the reasons leading to HCV infection have not been fully clearly understood yet, more and more evidences showed that cytokines obviously play important roles in perpetuating the chronic inflammatory state. TNF-α and TNF-β were also important mediators in the antiviral response.Citation43 In addition, data from related studies indicated the ability and degree to clear the virus, and the strength of T-cell response (such as TNF-α production), which correlated with the result of HCV infection.Citation31,44 There are significant differences on the ability to produce cytokines among individuals. Some studies suggested that the genetic polymorphisms of cytokine regulatory regions were one of the determining factors. The expression of cytokine genes is influenced by the polymorphisms of the genes when they were regulated.Citation45 We thought that the polymorphism of TNF-α is fairly important as it can be a sign to show the susceptibility or outcome of the infection, and to mediate the expressed level of TNF-α in different individuals. However, the result of ChenCitation46 suggested no significant association between TNF-α-308G/A polymorphism and susceptibility to HCV infection in the combined population. In order to get a more confident result, the meta-analysis was carried out in this study, in which the involved studies were increased to 15, with more lager sample than the previous analysis.Citation46 The focus of the current work was to comprehensively summarize the results of published studies of HCV infection and TNF-α polymorphisms, including the susceptibility as well as the spontaneous clearance of HCV. Besides, the analysis with the site of TNF-α-238 in our study was the first performed at present.
This meta-analysis showed no significant association between the TNF-α-308, -238 G/A polymorphisms and HCV infection/spontaneous clearance on global population. In order to study the impact of racial factor, we conducted subgroup study. In subgroup analysis, we also found the relationships mentioned above to be indefinite. Although no significant relationship was found between the polymorphisms of TNF-α-308, -238 and HCV infection/spontaneous clearance, we supposed that except for the reasons of living environment, pathogens (genotype and subtype of virus), the age and sex of host, and genetic factors are still important reasons for the outcome of HCV infection. However, we should realize that the genotype studied here represents only one factor among complex immune systems. The TNF-α serum levels were associated not only with the genetic factors but also with the host’s condition such as the basic disease and with the exposure to environmental pathogens. HCV infection is a complex disease which can be influenced by various kinds of factors. In addition, although meta-analysis would be helpful in revealing the relationship between polymorphism and phenotypes underlying the outcome of HCV infection, it is worth noting that it can directly describe the actual biological process. As a research strategy, meta-analysis serves as a tool for better understanding the associations in the real world. To sum up the reasons above, our meta-analysis and other studies, we should control the above factors as possible as we can, in order to study the further reasons for the susceptibility and the spontaneous clearance of HCV.
In conclusion, allelic variation at the -308, -238 sites of TNF-α was not significantly associated with HCV infection and spontaneous clearance, and the later result was first rendered by our present meta-analysis. Despite the negative results of our meta-analysis, we deemed it still played an essential role in other kinds of researches about HCV infection, and made an essential function in HCV prevention of HD patients.
Further studies are still needed to explore the potential effects of genetic variations on HCV infection in general patients and HD patients with larger samples and more different ethnic backgrounds.
ACKNOWLEDGMENTS
This work was supported by Children’s Miracle Network Awards 2005-10, Project supported by the Scientific Research Foundation of the State Human Resources Ministry for Selected Returned Overseas Chinese Scholars 2008-148, Project supported by the key lab of Jiangsu Province MMB09KF04, Project supported by the Scientific Research Program of the Health Department of Jiangsu Province H200902 and XK16200903, Project supported by the National Natural Science Foundation of China H0511-81070588, Project supported by the international cooperation projects of Jiangsu Province BZ2010058, and Project supported by the funding of blue project 2010.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sabahi A. Hepatitis C virus entry: The early steps in the viral replication cycle. Virol J. 2009;6:117.
- Hoofnagle J. Hepatitis C: The clinical spectrum of disease. Hepatology. 1997;26:15S.
- Sun J, Yu R, Zhu B, Wu J, Larsen S, Zhao W. Hepatitis C infection and related factors in hemodialysis patients in china: Systematic review and meta-analysis. Ren Fail. 2009;31:610–620.
- Ken CH, Qian X, Zhen Y. Investigation of hepatitis C virus infection in chronic hemodialysis patients. J Cell Mol Immunol. 2001;17(5):448–449 [in Chinese].
- Xiaonan Y, Li J, Weixin F, Hongfu G, Rongwei F. Study of hepatitis C virus infection in patients on hemodialysis. China Public Health. 2002;18(5):597–598 [in Chinese].
- Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009;4(1):207–220.
- McKenna O, Cunningham C, Blake C. Socio-demographic and clinical features of Irish iatrogenic hepatitis C patients: A cross-sectional survey. BMC Publ Health. 2009;9:323.
- Omland LH, Farkas DK, Jepsen P, Obel N, Pedersen L. Hepatitis C virus infection and risk of cancer: A population-based cohort study. Clin Epidemiol. 2010;2:179–186.
- Tilg H, Diehl AM. Cytokines in alcohlic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467.
- Saito I, Miyamura T, Ohbayashi A, . Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–6549.
- Di Bisceglie AM. Natural history of hepatitis C: Its impact in clinical management. Hepatology. 2000;31:1014–1018.
- Rao V, Fabrizi F, Pennell P, . Improved detection of hepatitis C virus infection by transcription-mediated amplification technology in dialysis population. Ren Fail. 2010;32(6):721–726.
- Koziel MJ. The role of immune responses in the pathogenesis of hepatitis C virus infection. J Viral Hepat. 1997;2:31–41.
- Fukuda R, Ishimura N, Ishihara S, . Intrahepatic expression of proinflammatory cytokine mRNAs and interferon efficacy in chronic hepatitis C. Liver. 1996;16:390–399.
- Larrea E, Garcia N, Qian C, . Tumor necrosis factor alpha gene expression and the response to interferon in chronic hepatitis C. Hepatology. 1996;23:210–217.
- Hall CH, Kassel R, Tacke RS, Hahn YS. HCV+ hepatocytes induce human regulatory CD4+ T cells through the production of TGF-beta. PLoS One. 2010;5(8):e12154.
- Persico M, Capasso M, Persico E, Interleukin-10-1082 GG polymorphism influences the occurrence and the clinical characteristics of hepatitis C virus infection. J Hepatol. 2006;45(6):779–785.
- Palmer C, Corpuz T, Guirguis M, . The effect of obesity on intrahepatic cytokine and chemokine expression in chronic hepatitis C infection. Gut. 2010;59(3):397–404.
- Pociot F, D’Alfonso S, Compasso S, . Functional analysis of a new polymorphism in the human TNF-α gene promoter. Scand J Immunol. 1995;42:501e4.
- Wilson AG, di Giovine FS, Blakemore AI, Single base polymorphism in the human tumor necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet. 1992;1:353.
- Constantini PK, Wawrzynowicz-Syczewska M, Clare M, Interleukin-1, interleukin-10 and tumour necrosis factor-alpha gene polymorphisms in hepatitis C virus infection: An investigation of the relationships with spontaneous viral clearance and response to alpha-interferon therapy. Liver. 2002;22(5):404–412.
- Xiao-rong M, Hong Y. Genetic association of tumor necrosis factor-alpha polymorphism with hepatits C virus infection. Clin Focus. 2007;22:6 [in Chinese].
- Goyal A, Kazim SN, Sakhuja P, . Association of TNF-beta polymorphism with disease severity among patients infected with hepatitis C virus. J Med Virol. 2004;72(1):60–65.
- Zein NN, Germer JJ, El-Zayadi AR, . Ethnic differences in polymorphisms of tumor necrosis factor-alpha, interleukin-10, and transforming growth factor-beta1 genes in patients with chronic hepatitis C virus infection. Am J Trop Med Hyg. 2004;70(4):434–437.
- Lio D, Caruso C, Di Stefano R, IL-10 and TNF-alpha polymorphisms and the recovery from HCV infection. Hum Immunol. 2003;64(7):674–680.
- Mangia A, Santoro R, Piattelli M, IL-10 haplotypes as possible predictors of spontaneous clearance of HCV infection. Cytokine. 2004;25(3):103–109.
- Hohler T, Kruger A, Gerken G, . Tumor necrosis factor alpha promoter polymorphism at position -238 is associated with chronic active hepatitis C infection. J Med Virol. 1998;54(3):173–177.
- Bahr MJ, el Menuawy M, Boeker KH, . Cytokine gene polymorphisms and the susceptibility to liver cirrhosis in patients with chronic hepatitis C. Liver Int. 2003;23(6):420–425.
- Wang Y, Kato N, Hoshida Y, Interleukin-1beta gene polymorphisms associated with hepatocellular carcinoma in hepatitis C virus infection. Hepatology. 2003;37(1):65–71.
- Rosen HR, McHutchison JG, Conrad AJ, . Tumor necrosis factor genetic polymorphisms and response to antiviral therapy in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97(3):714–720.
- Rosen HR, Miner C, Lewinsohn D, . Determination of hepatitis C virus-specific effector/memory CD4_T cell frequencies at different clinical stages of disease. Hepatology. 2002;35:190–198.
- Schiemann U, Glas J, Torok P, . Response to combination therapy with interferon alfa-2a and ribavirin in chronic hepatitis C according to a TNF-alpha promoter polymorphism. Digestion. 2003;68(1):1–4.
- Valenti L, Pulixi E, Fracanzani AL, . TNFalpha genotype affects TNFalpha release, insulin sensitivity and the severity of liver disease in HCV chronic hepatitis. J Hepatol. 2005;43(6):944–950.
- Kusumoto K, Uto H, Hayashi K, Interleukin-10 or tumor necrosis factor-alpha polymorphisms and the natural course of hepatitis C virus infection in a hyperendemic area of Japan. Cytokine. 2006;34(1–2):24–31.
- Barrett S, Collins M, Kenny C, . Polymorphisms in tumour necrosis factor-alpha, transforming growth factor-beta, interleukin-10, interleukin-6, interferon-gamma, and outcome of hepatitis C virus infection. J Med Virol. 2003;71(2):212–218.
- Pereira FA, Pinheiro da Silva NN, Rodart IF, Association of TGF-beta1 codon 25 (G915C) polymorphism with hepatitis C virus infection. J Med Virol. 2008;80(1):58–64.
- Yanjuan ZH, Lvtan L. Detection of hepatitis C virus marker in hemodialysis patients. Chin J Nephrol. 1997;13(5):283 [in Chinese].
- Wang C, Sun J, Zhu B, . Hepatitis B virus infection and related factors in hemodialysis patients in China – Systematic review and meta-analysis. Ren Fail. 2010;32(10):1255–1264.
- Mioli VA, Balestra E, Bibiano L, . Epidemiology of viral hepatitis in dialysis patients centers: A national survey. Nephron. 1992;61(3):278–283.
- Fabrizi F, Lunghi G, Bacchini G, . Hepatitis G virus infection in chronic dialysis patients and kidney transplant recipients. Nephron Dial Transplant. 1997;12(8):1645–1651.
- Xijie L, Liuqin L. Relationship between hemodialysis and viral hepatitis. J Nephrol Dialy Transplant. 1998;7(3):245–246 [in Chinese].
- Xinyu ZH, Yulin G, Houyi W, Lvqi H. Infection of hepatitis B, C and G viruses in hemodialysis patients. J Clin Intern Med. 2001;18(3):214–216 [in Chinese].
- Koziel MJ. Cytokines in viral hepatitis. Sem Liver Dis. 1999;19:157–169.
- Takaki A, Wiese M, Maertens G, . Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582.
- Knight JC, Udalova I, Hill AV, . A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nat Genet. 1999;22:145–150.
- Chen Y, Pei J. An assessment of a TNF polymorphic marker for the risk of HCV infection: Meta-analysis and a new clinical study design. Infect Genet Evol. 2009;9(6):1356–1363.