Abstract
Background: T helper 1 (Th1)/T helper 2 (Th2) profile is pivotal in the development of fibrosis. Renal interstitial fibroblasts, which play a central role in the development of renal interstitial fibrosis, have an intimate relation with lymphocytes. However, there is little knowledge of the effect of fibroblasts on the profile of CD4 T-lymphocyte subsets. Methods: After coculture with rat renal interstitial fibroblasts, the proportions of Th1 and Th2 cells in CD4 T lymphocytes and the apoptosis rates of the two subsets were detected by flow cytometry. Galectin-9 expression in rat renal interstitial fibroblasts was detected by immunofluorescence, Western blotting, and enzyme-linked immunosorbent assay. Results: After 48 h of coculture, rat renal interstitial fibroblasts increased the proportion of Th2 cells, lowering the ratio of Th1/Th2. Meanwhile, interferon-gamma production in Th1 cells was inhibited and interleukin-4 production in Th2 was promoted. After coculture with activated rat renal interstitial fibroblasts for 24 h, apoptosis of Th1 was more highly promoted than that of Th2 cells. In addition, rat renal interstitial fibroblasts induced stronger Th2 cell differentiation than that of Th1 cells in vitro. Rat renal interstitial fibroblasts expressed but did not secrete galectin-9 (an apoptosis-inducing factor for Th1 cells) in vitro and the expression level decreased when cocultured with CD4 T lymphocytes. Conclusions: Rat renal interstitial fibroblasts shift the Th1/Th2 profile in vitro, and this may be another pathway by which renal interstitial fibroblasts promote fibrosis.
INTRODUCTION
Many types of progressive renal diseases (such as those caused by infection, immune disorders, drugs, poisons, or obstruction factors) can result in renal tubulointerstitial fibrosis.Citation1 Although fibrosis is a normal physiological repair process in response to persisting tissue injury, if left unchecked it can lead to abnormal tissue remodeling and permanent scarring. The severity of interstitial fibrosis is an accurate predictor of kidney survival.Citation2 The mechanism of fibrosis is similar regardless of etiology. During chronic injury or chronic inflammation in renal, the sustained activation of myofibroblasts was induced and leads to deposition of fibrous tissue. Interstitial myofibroblasts have been proposed to originate from five sources: resident fibroblasts, adventitial fibroblasts, circulating fibrocytes, tubular epithelial–mesenchymal transition, or endothelial/mesenchymal transition. However, resident renal interstitial fibroblasts are still considered as the most important component.Citation3,4
T lymphocytes can modulate the fibrogenic process by direct interaction with myofibroblasts and secreting cytokines.Citation5 In general, T helper 1 (Th1) lymphocytes, expressing high levels of antifibrotic cytokines such as interferon-gamma (IFN-γ), have an antifibrotic role, whereas T helper 2 (Th2) lymphocytes, expressing high levels of profibrotic cytokines such as interleukin (IL)-4 and IL-13, have a profibrotic role.Citation5–7 Therefore, the Th1/Th2 profile is pivotal for the development of fibrosis and may be a therapeutic target. The mechanism involved in the regulation of Th1/Th2 balance during fibrogenesis is unknown.
The activation and function of myofibroblasts is regulated by immune cells.Citation5,6 Meanwhile, activated myofibroblasts can influence lymphocyte infiltration, activation, and proliferation by secreting all kinds of proinflammatory cytokines and chemokines to promote the development of chronic inflammation and fibrosis.Citation8,9So far, there have been no reported studies investigating the effect of myofibroblasts on the profile of CD4 T-lymphocyte subsets.
In this study, we performed coculture of CD4 T lymphocytes and rat renal interstitial fibroblasts in vitro to determine whether rat renal interstitial fibroblasts can change the Th1/Th2 profile and explored the potential mechanism. Galectin-9 induces Th1 cell apoptosis by combining with T cell immunoglobulin mucin 3 (Tim-3) on the surface of Th1 cells.Citation10 It was found expressed in human lung fibroblasts,Citation11 dermal fibroblasts, Citation12 and fibroblasts in liver with drug-induced injury.Citation13 So we wonder if rat renal interstitial fibroblasts promote apoptosis of Th1 by secreting galectin-9 and then analyzed galectin-9 expression in rat renal interstitial fibroblasts.
MATERIALS AND METHODS
Preparation of Rat Renal Interstitial Fibroblasts
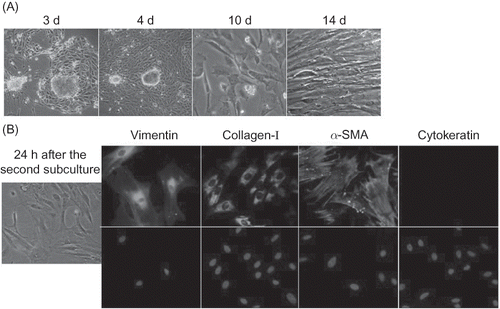
Rat renal interstitial fibroblasts were prepared through explantation of renal interstitial tissue in vitro as described previously.Citation14 The medium used was RPMI 1640 (Gibco, Grand Island, NY, USA) containing 20% fetal bovine serum (FBS) (Gibco). There would be some cubic or polygonal kidney tubular epithelial cells mixed in the primary culture. Pure interstitial fibroblasts could be obtained after two subcultures. Anti-vimentin (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-collagen-I (1:500; Abcam, Cambridge, UK), anti-α-smooth muscle actin (SMA) (1:800; Abcam), and anti-pan-cytokeratin (1:100; Santa Cruz Biotechnology) antibodies, as well as fluorescein isothiocyanate (FITC)-conjugated anti-rabbit (1:200; Zhongshan-Golden Bridge, Beijing, PR China), anti-mouse (1:500; Abcam), or anti-goat (1:40; MBL, Xiamen, PR China) secondary antibodies, were used to identify renal interstitial fibroblasts by the immunofluorescence technique.Citation14
Preparation of Rat CD4 T Lymphocytes
Rat spleen lymphocytes were isolated as described previouslyCitation15 and cultured in RPMI 1640 containing 10% FBS for 2 h to remove any adhering monocytes. Rat CD4 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were used to obtain CD4 T lymphocytes through magnetic cell sorting according to the manufacturer’s guidelines. The cell purity detected by flow cytometry was 98.23 ± 0.27%.Citation15 Anti-CD4-FITC was from Becton Dickinson (BD) (Franklin Lakes, NJ, USA).
Coculture of Rat CD4 T Lymphocytes and Renal Interstitial Fibroblasts
After culturing for 24 h in 6-well culture plates, the medium of renal interstitial fibroblasts (2 × 105 cells/well) was changed for the coculture with freshly isolated CD4 T lymphocytes (4 × 106 cells/well) using Millipore Hanging Cell Culture Inserts (0.4 μm; Millipore, Billerica, MA, USA) in RPMI 1640 containing 10% FBS. We performed coculture without adding any lymphocyte mitogens (the reason in the Discussion section). CD4 T lymphocytes cultured alone were used as the control. During each experiment, the lymphocytes (or fibroblasts) were from the same rat and one rat was only used for one experiment.
A carboxyfluorescein diacetate succinimidyl ester cell proliferation assay (Invitrogen, Carlsbad, CA, USA) was used to determine whether CD4 T lymphocytes could proliferate in vitro in the absence of mitogens. An Annexin V/PI assay was used to determine the proportion of viable cells within the CD4 T-lymphocyte population by flow cytometry using a 488 Annexin V/Dead Cell Apoptosis Kit (Invitrogen). The double-negative cells (i.e., cells which were negative for both Annexin V and PI) were considered viable.
Rat renal interstitial fibroblasts (5 × 103 cells/well) were cultured alone or cocultured with CD4 T lymphocytes (5 × 104 cells/well) (as described above) in 96-well culture plates. At the beginning of the coculture and at the end of 24 h or 48 h of coculture, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed to detect the proliferation of rat renal interstitial fibroblasts as described previously.Citation16
Flow Cytometry Analysis for Determining Proportions of Th1 and Th2 Cells in CD4 T Lymphocytes and Apoptosis Rates of Th1 and Th2 Cells
After 48 h of coculture, the proportions of Th1 cells (IFN-γ-positive cells) and Th2 cells (IL-4-positive cells) in CD4 T lymphocytes were detected by flow cytometry (FCM) analysis using anti-IFN-γ-phycoerythrin (PE) (BD) and anti-IL-4-PE antibodies (BD).Citation15 The isotype control antibody used was from BD Company.
After 24 h of coculture, a caspase-3 staining procedure was performed on CD4 T lymphocytes with the Fluorescein Active Caspase-3 Staining Kit (Biovision, Mountain View, CA, USA) according to the manufacturer’s protocol before staining IFN-γ and IL-4 to determine the apoptosis rates of Th1 and Th2 cells.
Detection of Galectin-9 Expression in Rat Renal Interstitial Fibroblasts
Galectin-9 expression in rat renal interstitial fibroblasts was detected by immunofluorescence as described previously,Citation14 and Western blot analysis was used to determine whether coculture with CD4 T lymphocytes had effect on the galectin-9 expression.Citation11 The antibodies used in the immunofluorescence analysis were anti-galectin-9 antibody (1:200; Abcam) and FITC-conjugated goat anti-rabbit secondary antibody (1:200; Zhongshan-Golden Bridge). The materials used for the Western blot analysis were anti-galectin-9 (1:1000; Abcam) and anti-β-actin (1:4000; Sigma, St. Louis, MO, USA) antibodies and horseradish peroxidase-conjugated anti-rabbit (1:9000) or anti-mouse (1:8000; both from Zhongshan-Golden Bridge) secondary antibodies. An enzyme-linked immunosorbent assay (ELISA) kit for rat galectin-9 (Uscn Life Science, Wuhan, PR China) was used to detect galectin-9 in the supernatant of rat renal interstitial fibroblasts cultured alone or cocultured with CD4 T lymphocytes according to the manufacturer’s guidelines.
An In Vitro Study to Examine the Effect of Rat Renal Interstitial Fibroblasts on the Differentiation of Th1 and Th2 Cells
A type of CD4 T lymphocyte positive in CD25 without IFN-γ or IL-4 expression and with differentiation potency was obtained by culturing the freshly isolated CD4 T lymphocytes in culture medium with 5 μg/mL concanavalin A (Con A) for 2 weeks. The medium of these lymphocytes was changed and the lymphocytes were cocultured with rat renal interstitial fibroblasts in 6-well culture plates as described above. After 48 h of coculture, proportions of Th1 and Th2 in CD4 T cells were detected by FCM. For each experiment, the lymphocytes (or fibroblasts) were from the same rat and one rat was only used for one experiment.
Statistics
Data were analyzed using SPSS 14.0 (SPSS, Chicago, IL, USA). Paired t-test and randomized block design analysis of variance were applied, and p < 0.05 was considered statistically significant. The data in graphs were expressed as mean ± SD.
RESULTS
Identification of Rat Renal Interstitial Fibroblasts
After 3 days of culture it was observed that spindle cells had grown around some tissue pieces and begun to proliferate. One week later, the cells enlarged and stretched out to form pseudopodia. After 2 weeks, the cells were about 75% confluent (A). After two subcultures, pure rat renal interstitial fibroblasts were obtained, which were positive for vimentin, α-SMA, and collagen-I, and negative for cytokeratin (an epithelial marker) (B). The positivity for α-SMA suggested that the cells had characteristics of myofibroblasts.Citation17
General Effects of Coculture on Viability of the Two Kinds of Cells
As the basis of our research, we first examined whether there were any effects on viability of the two cells when coculture was performed. After coculture with CD4 T cells for 48 h, rat renal interstitial fibroblasts had no changes in morphology and their proliferation rate detected by MTT assay only mildly increased compared with that of those cultured alone (A). In the absence of a lymphocyte, mitogen CD4 T cells that were cultured alone or cocultured with renal interstitial fibroblasts could not proliferate and gradually underwent apoptosis. When cocultured with rat renal interstitial fibroblasts, apoptosis of CD4 T cells increased. After 48 h of coculture, the proportion of viable cells in CD4 T lymphocytes which were cocultured with rat renal interstitial fibroblasts was significantly lower than that in CD4 T lymphocytes cultured alone (B).
Coculture with Rat Renal Interstitial Fibroblasts Changed the Th1/Th2 Profile
At the end of the 48 h coculture with rat renal interstitial fibroblasts, in the coculture group the proportion of Th2 cells increased compared with the control, while the proportion of Th1 cells was similar to that of the control (p = 0.69), and the Th1/Th2 ratio decreased (p < 0.05) (A). After coculture with rat renal interstitial fibroblasts for 48 h, the mean fluorescence intensity of IFN-γ in Th1 cells decreased, but that of IL-4 in Th2 cells increased (B).
Apoptosis Was More Strongly Enhanced in Th1 Cells than in Th2 Cells When Cocultured with Rat Renal Interstitial Fibroblasts
After coculture with rat renal interstitial fibroblasts for 24 h, apoptosis rates of Th1 and Th2 cells in the coculture group were both higher than those in the control group. Although apoptosis of both Th1 and Th2 cells increased in the coculture group, the increase of apoptosis in Th1 cells was significantly higher than in Th2 cells, suggesting that Th1 cell apoptosis was more highly advanced ().
Figure 2 General effects of coculture on the viability of the two kinds of cells. (A) The proliferation rates of rat renal interstitial fibroblasts cultured alone and those cocultured with rat CD4 T lymphocytes for 24 or 48 h were measured by MTT assay (n = 3). (B) Proportions of viable cells within CD4 T lymphocytes cultured alone and those cocultured with rat renal interstitial fibroblasts (48 h) were determined with an Annexin V/PI assay (n = 3). All data were expressed as mean ± SD.
Notes: *p < 0.05 for data of the cocultured fibroblasts compared with the cultured alone. **p < 0.01 for data of the cocultured lymphocytes compared with the cultured alone (the control).
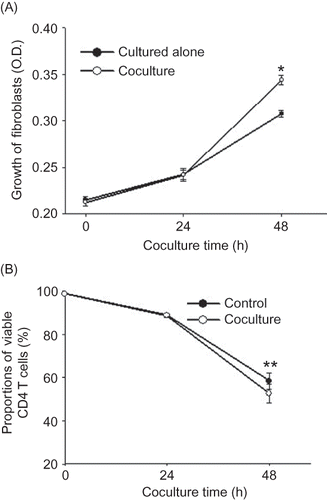
Figure 3 Coculture with rat renal interstitial fibroblasts changed the Th1/Th2 profile. (A) FCM analysis of the proportions of Th1 and Th2 cells within CD4 T lymphocytes in the control and coculture groups. (B) FCM analysis of mean fluorescence intensity of IFN-γ in Th1 cells and IL-4 in Th2 cells in the control and coculture groups (48 h). Control group: rat CD4 T lymphocytes cultured alone; coculture group: rat CD4 T lymphocytes cocultured with rat renal interstitial fibroblasts for 48 h (n = 10). Data were expressed as mean ± SD.
Note: *p < 0.05 and **p < 0.01 for data compared with the control.
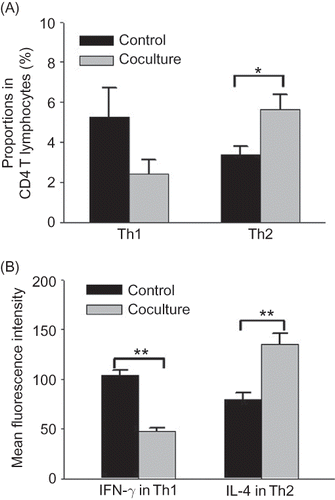
Figure 4 Effect of coculture with rat renal interstitial fibroblasts on apoptosis of Th1 and Th2 cells. FCM analysis of apoptosis rates in Th1 and Th2 cells in the control and coculture groups (24 h) using caspase-3 staining. Control group: rat CD4 T lymphocytes cultured alone; coculture group: rat CD4 T lymphocytes cocultured with rat renal interstitial fibroblasts for 24 h (n = 10). Data were expressed as mean ± SD.
Notes: *p < 0.01 compared with the control data. **p < 0.01 for data compared between any two CD4+ T-lymphocyte subsets.
Figure 5 Galectin-9 expression in rat renal interstitial fibroblasts. (A) Immunofluorescence analysis of galectin-9 expressed in rat renal interstitial fibroblasts (400× magnification). (B) Western blot analysis of galectin-9 expression in rat renal interstitial fibroblasts cultured alone and those cocultured with CD4 T lymphocytes for 24 or 48 h (n = 5). Data were expressed as mean ± SD.
Note: *p < 0.01 for data of the cocultured fibroblasts compared with the cultured alone.
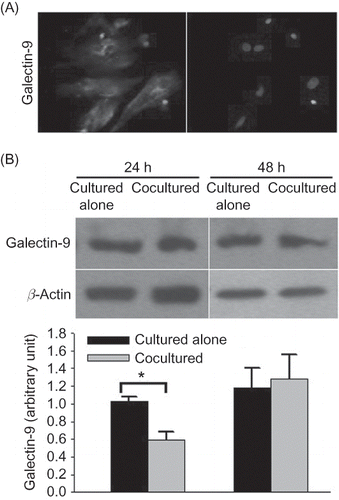
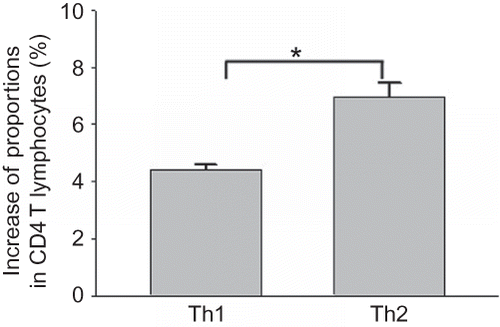
Figure 6 Effect of coculture with rat renal interstitial fibroblasts on the differentiation of Th1 and Th2 cells. FCM analysis showing the increase in proportions of IFN-γ-positive cells (Th1 cells) and IL-4-positive cells (Th2 cells) after CD4 T lymphocytes positive in CD25 without IFN-γ or IL-4 expression and with differentiation potency were cocultured with renal interstitial fibroblasts for 48 h (n = 5). Data were expressed as mean ± SD.
Note: *p < 0.01 for data compared between Th1 cells and Th2 cells.
Expression of Galectin-9 in Rat Renal Interstitial Fibroblasts
We found that rat renal interstitial fibroblasts expressed galectin-9 through immunofluorescence analysis (A). When cocultured with freshly isolated CD4 T lymphocytes for 24 h, galectin-9 expression in the rat renal interstitial fibroblasts decreased compared with that in fibroblasts cultured alone, but this phenomenon was not found when cocultured for 48 h (p = 0.213) (B). However, galectin-9 was undetectable by ELISA in the supernatant of rat renal interstitial fibroblasts cultured alone or cocultured with CD4 T lymphocytes.
Rat Renal Interstitial Fibroblasts Induced Th2 Cell Differentiation More Strongly than Th1 Cell Differentiation
CD4 T lymphocytes positive in CD25 without IFN-γ or IL-4 expression and with differentiation potency were cocultured with rat renal interstitial fibroblasts for 48 h, and proportions of both IFN-γ-positive cells (Th1 cells) and IL-4-positive cells (Th2 cells) increased significantly. However, the increase was significantly higher in Th2 cells than in Th1 cells, suggesting that the rat renal interstitial fibroblasts induce Th2 cell differentiation to a greater extent than Th1 cell differentiation in vitro ().
DISCUSSION
Activated myofibroblasts can regulate immune cells to promote chronic inflammation through a variety of ways.Citation8,9,18 There are two-way effects between myofibroblasts and immune cells. Many cytokines from leukocytes can also promote activation of myofibroblasts, and repeated activation of myofibroblasts with a proinflammatory role results in sustained chronic inflammation and fibrosis.Citation6 In this study, we cocultured freshly isolated rat CD4 T lymphocytes and rat renal interstitial fibroblasts with neither direct contact nor any mitogens to determine the effect of rat renal interstitial fibroblasts on Th1/Th2 profile. Here, we give an explanation for why we performed coculture without adding any lymphocyte mitogens. As described previously,Citation19 we found that in vitro a lymphocyte mitogen (such as Con A) induced CD4 T lymphocytes to activate into a type of lymphocyte with reproductive activity, which was positive in CD25 but did not express IFN-γ or IL-4 (no results were shown), preventing our observation on the change of Th1/Th2 profile. We found that coculture with rat renal interstitial fibroblasts increased the proportion of Th2 cells in CD4 T lymphocytes, lowering the Th1/Th2 ratio. This alteration is possibly caused by more highly enhanced apoptosis in Th1 than in Th2 cells when cocultured with rat renal interstitial fibroblasts, as found in this study. Meanwhile, the function of Th1 and Th2 lymphocytes was influenced when cocultured with renal interstitial fibroblasts. After coculture with renal interstitial fibroblasts for 48 h, the mean fluorescence intensity of IFN-γ in Th1 cells decreased and that of IL-4 in Th2 cells increased, suggesting that the IL-4-producing function of Th2 was enhanced and the IFN-γ-producing function of Th1 was inhibited.
It is thought that Th2 lymphocytes, expressing high levels of profibrotic cytokines (e.g., IL-4, IL-13), have a profibrotic role, whereas Th1 lymphocytes, expressing high levels of antifibrotic cytokines (e.g., IFN-γ), have an antifibrotic role.Citation5–7 IFN-γ can reduce renal fibrosis and preserved renal function through a strong inhibition of myofibroblasts in a rat subtotal nephrectomy model,Citation20 and Th1 cells can inhibit the differentiation and maturation of Th17 cells and Th2 cells by secreting IFN-γCitation21,22 and prevent the proinflammatory and profibrogenic effect of Th17 cells and Th2 cells. In addition, Th1 response can drive the differentiation of the so-called M1 macrophages, thereby avoiding overgeneration of M2 macrophages with a profibrogenic role.Citation23 Therefore, renal interstitial fibroblasts might participate in the modulation of the Th1/Th2 profile to promote chronic inflammation and fibrosis.
It is mainly through the secretion of all kinds of cytokines that myofibroblasts affect lymphocytes to promote chronic inflammation.Citation8,9 We performed coculture with hanging cell culture inserts without any direct contact between the two cells to remove various factors caused by direct contact and to only detect the effect of rat renal interstitial fibroblasts on CD4 T lymphocytes by secreting cytokines. Galectin-9, a ligand of Tim-3, can induce the apoptosis of Th1 cells through binding Tim-3 on the surface of Th1 cells.Citation10 It was found that galectin-9 was expressed in fibroblasts from some other organs.Citation11–13 In this study, we found that rat renal interstitial fibroblasts expressed galectin-9 and the expression was not influenced when cocultured with CD4 T lymphocytes. However, it seems that rat renal interstitial fibroblasts were unable to secrete galectin-9 in vitro according to the ELISA result. These findings do not support the hypothesis that rat renal interstitial fibroblasts induce Th1 cell apoptosis by secreting galectin-9. Further researches may be needed to ascertain whether other factors can induce galectin-9 secretion by renal interstitial fibroblasts.
In addition, when CD4 T lymphocytes positive in CD25 without IFN-γ or IL-4 expression and with differentiation potency were cocultured with rat renal interstitial fibroblasts, the increase in proportion of Th2 cells was significantly higher than that of Th1 cells, suggesting that Th2 cell differentiation was induced to a greater extent than that of Th1 cells. So, rat renal interstitial fibroblasts can also modulate Th1/Th2 balance through the effect on their differentiation.
There were two effects of CD4 T lymphocytes on rat renal interstitial fibroblasts in this study. First, we observed that CD4 T lymphocytes promoted the proliferation of rat renal interstitial fibroblasts during coculture. It was reported that some chemical mediators from apoptotic cells and phagocytosis of apoptotic body could promote the activation and function of myofibroblasts in injured liver.Citation24 We performed coculture without adding any lymphocyte mitogens. In the absence of a mitogen, CD4 T cells cultured in vitro could not proliferate and gradually underwent apoptosis. However, whether the proliferation of rat renal interstitial fibroblasts was promoted by apoptosis of lymphocytes remains uncertain. Second, galectin-9 expression in rat renal interstitial fibroblasts decreased after coculture with freshly isolated CD4 T lymphocytes for 24 h, but the reason is unclear. In the previous studies, it was showed that IFN-γ and other inflammatory cytokines upregulated galectin-9 expression in stromal cells such as fibroblasts and endothelial cells,Citation11,12,25 which cannot help explain this result. We think that maybe some cytokines from CD4 T lymphocytes can inhibit galectin-9 expression in myofibroblasts, and due to the reduced quantity of CD4 T lymphocytes there was no difference found when cocultured for 48 h.
In summary, in vitro rat renal interstitial fibroblasts can increase the apoptosis of Th1 cells more highly than that of Th2 cells, decrease the Th1/Th2 ratio, inhibit the IFN-γ-producing function of Th1 cells, and promote the IL-4-producing function of Th2 cells, and also preferentially induce Th2 differentiation. According to the knowledge from previous studies, it can be considered that these changes would possibly promote chronic inflammation and fibrosis.
LIMITATIONS
This is only a preliminary in vitro study into the single effect of rat renal interstitial fibroblasts on the profile of Th1 and Th2 cells, and the rapid apoptosis of CD4 T lymphocytes during in vitro culture without lymphocyte mitogens also provides a limitation of the research. The results we obtained may not be completely consistent with the in vivo condition, which is more complicated and would be influenced by many other factors. Further researches, including in vivo studies, are needed to verify our results and explore the mechanism involved in these phenomena.
ACKNOWLEDGMENT
We thank Xu Yong for assistance with FCM analysis and Aiting Yang, Tianhui Liu, Ping Wang, and Min Cong for their experimental technique support and helpful comments.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Harris RC, Neilson EG. Toward a unified theory of renal progression. Annu Rev Med. 2006;57:365–380.
- Bohle A, Strutz F, Muller GA. On the pathogenesis of chronic renal failure in primary glomerulopathies: A view from the interstitium. Exp Nephrol. 1994;2:205–210.
- Grgic I, Duffield JS, Humphreys BD. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr Nephrol. 2011. (in press).
- Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13:279–284.
- Marra F, Aleffi S, Galastri S, Provenzano A. Mononuclear cells in liver fibrosis. Semin Immunopathol. 2009;31:345–358.
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210.
- Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333.
- Parsonage G, Falciani F, Burman A, . Global gene expression profiles in fibroblasts from synovial, skin and lymphoid tissue reveals distinct cytokine and chemokine expression patterns. Thromb Hemost. 2003;90:688–697.
- Buckley CD, Amft N, Bradfield PF, . Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423–3429.
- Zhu C, Anderson AC, Schubart A, . The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252.
- Asakura H, Kashio Y, Nakamura K, . Selective eosinophil adhesion to fibroblast via IFN-gamma-induced galectin-9. J Immunol. 2002;169:5912–5918.
- Igawa K, Satoh T, Hirashima M, Yokozeki H. Regulatory mechanisms of galectin-9 and eotaxin-3 synthesis in epidermal keratinocytes: Possible involvement of galectin-9 in dermal eosinophilia of Th1-polarized skin inflammation. Allergy. 2006;61:1385–1391.
- Takahashi Y, Fukusato T, Kobayashi Y, . High expression of eosinophil chemoattractant ecalectin/galectin-9 in drug-induced liver injury. Liver Int. 2006;26:106–115.
- Grimwood L, Masterson R. Propagation and culture of renal fibroblasts. Methods Mol Biol. 2009;466:25–37.
- Caraher EM, Parenteau M, Gruber H, Scott FW. Flow cytometric analysis of intracellular IFN-gamma, IL-4 and IL-10 in CD3(+)4(+) T-cells from rat spleen. J Immunol Methods. 2000;244:29–40.
- Ku BM, Ryu HW, Lee YK, . 4′-Acetoamido-4-hydroxychalcone, a chalcone derivative, inhibits glioma growth and invasion through regulation of the tropomyosin 1 gene. Biochem Biophys Res Commun. 2010;402:525–530.
- Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283.
- Pilling D, Akbar AN, Girdlestone J, . Interferon-beta mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–1050.
- Chen JF, Gao J, Zhang D, Wang ZH, Zhu JY. CD4+Foxp3+ regulatory T cells converted by rapamycin from peripheral CD4+CD25(–) naive T cells display more potent regulatory ability in vitro. Chin Med J (Engl). 2010;123:942–948.
- Oldroyd SD, Thomas GL, Gabbiani G, El Nahas AM. Interferon-gamma inhibits experimental renal fibrosis. Kidney Int. 1999;56:2116–2127.
- Harrington LE, Hatton RD, Mangan PR, . Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132.
- Park H, Li Z, Yang XO, . A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141.
- McGaha TL, Bona CA. Role of profibrogenic cytokines secreted by T cells in fibrotic processes in scleroderma. Autoimmun Rev. 2002;1:174–181.
- Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30:402–410.
- Imaizumi T, Kumagai M, Sasaki N, . Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J Leukoc Biol. 2002;72:486–491.