Abstract
This study was conducted to evaluate the effects of Zingiber officinale Roscoe (Ginger), Arabic gum (AG), and Boswellia on both acute and chronic renal failure (CRF) and the mechanisms underlying their effects. Acute renal failure was induced by 30 min ischemia followed by 24 h reperfusion, while CRF was induced by adenine feeding for 8 weeks. Prophylactic oral administration of ginger, AG, Boswellia, or vehicle (in control groups) was started 3 days before and along with adenine feeding in different groups or 7 days before ischemia–reperfusion. Ginger and AG showed renoprotective effects in both models of renal failure. These protective effects may be attributed at least in part to their anti-inflammatory properties as evident by attenuating serum C-reactive protein levels and antioxidant effects as evident by attenuating lipid peroxidation marker, malondialdehyde levels, and increasing renal superoxide dismutase activity. Ginger was more potent than AG in both models of renal failure. However, Boswellia showed only partial protective effect against both acute renal failure and CRF and it had no antioxidant effects. Finally, we can say that ginger and AG could be beneficial adjuvant therapy in patients with acute renal failure and CRF to prevent disease progression and delay the need for renal replacement therapy.
INTRODUCTION
It was reported that by 2010 there will be more than 2 million patients worldwide on maintenance dialysis, a 400% increase in 20 years. This increase, occurring predominantly in developing nations, is being driven especially by a worldwide increase in the incidence of diabetes and is too great to be offset by increased rates of renal transplantation.Citation1 The social and financial costs of renal replacement therapy are proving too great for developed nations to cope with and are an impossible burden for developing nations to meet.Citation2
Human kidneys are under assault from environmental toxins as well as from an array of diseases that place them at risk of ultimately failing. Several natural products have been used to protect against drug-induced toxicity and carcinogenic xenobiotics. Herbs and spices are generally considered safe and proved to be effective against various human ailments and their medicinal uses have been gradually increased in the developed countries.
Zingiber officinale Roscoe (Ginger), an indispensable component of curry, belongs to the Zingiberaceae family. Ginger has been used to treat a number of diseases, including headache, cold, arthritis, postoperative nausea, vomiting, and motion sickness, and reduces symptoms in patients with nausea of pregnancy.Citation3–6
Boswellia carteri is one of the 43 species in the genus Boswellia of the family Burseraceae. It is easily available in the United States as dietary supplements for patients with arthritis or other inflammation and pain-related disorders but its mechanisms of action are not well understood, and only a few studies of its analgesic, antihyperalgesic, and anti-inflammatory effects on animal models have been reported.Citation7
Arabic gum (AG) is dried gummy exudates from the stems and branches of Acacia senegal (Leguminosae).Citation8 AG has long been used in Arab folk medicine to reduce both the frequency and the need for hemodialysis in patients with chronic renal failure (CRF). Additionally, AG has been shown to reduce urinary nitrogen excretion by increasing urea disposal in the cecum and lowers serum urea concentration in rat and human.Citation9 However, there is a dearth of information providing scientific support for the folkloric claims of AG on renal function after renal damage.
In this study, we examined and compared the effects of ginger, AG, and Boswellia on renal function impairment induced by feeding adenine or ischemia–reperfusion (IR) injury. In addition, the possible underlying mechanisms were also investigated.
MATERIALS AND METHODS
All experiments were carried out in accordance with protocols approved by the local experimental ethics committee and in accordance with the guidelines of the US National Institutes of Health on animal care. Every effort was made to minimize the number of animals used and their suffering.
Animals
Adult male albino rats weighing about 220–250 g were obtained from the Faculty of Veterinary Medicine, Zagazig University, Egypt. The animals were housed in stainless steel cages with wood shaving bedding. They were kept at constant temperature of 25 ± 2°C, relative humidity of approximately 50%, and illumination (12 h light/dark) throughout the experiment. They were fed standard chow diet and were given free access to tap water.
Chemicals
Adenine was obtained from Sigma (St. Louis, MO, USA) and was prepared freshly in 10% Tween 80 obtained from El Gomhouria Co., Zagazig, Egypt. Ginger, Boswellia, and AG were obtained from the local source. Ginger and AG were dissolved in distilled water, but Boswellia was dissolved in sesame oil just before use. Kits for measuring serum creatinine and urea were supplied by Egyptian Company for Biotechnology, Egypt; for lactate dehydrogenase (LDH) obtained from biodiagnostic kit supplied by Spinreact, Spain; for malondialdehyde (MDA) supplied by Bio Diagnostic Co., Egypt; for CRP kit supplied by DiaMed EuroGen, Belgium; and for superoxide dismutase (SOD) from diagnostic kit, Bio Diagnostic Co.
Induction of Chronic Renal Failure
CRF was induced by oral administration of adenine in a dose of 200 mg/kg three times a week for 8 weeks suspended in 10% Tween 80.Citation10
Induction of Acute Renal Failure
Renal ischemia–reperfusion (RIR) was performed according to the method described by Rabb et al.Citation11 Rats were anesthetized with an intraperitoneal injection of thiopental sodium (50 mg/kg). To perform nephrectomy, a lateral incision was made in the left side and the left kidney was mobilized to allow left renal artery to be ligated, then this kidney was removed. The wound was closed with 3-0 silk suture for internal muscles and 4-0 silk suture for external skin. To cause ischemia, a lateral incision was made in the right side and the right kidney was mobilized and the right renal artery was clamped. This clamping was performed by tying the right renal artery over a plastic support for 30 min. Ischemia was confirmed visually by blanching of the kidney. After 30 min the tie was cut to eliminate the clamp and the wound was closed as described before. The animals were then returned to their cages and allowed free access to food and water after intramuscular injection with 0.5 mL penicillin. Animals were killed 24 h later. Sham-operated animals underwent the same operation but without left kidney removal or right renal artery clamping.
Experimental Design
One week after acclimatization, rats were randomly divided into 10 groups (10 rats each). Group I includes control rats given only 10% Tween 80 by oral gavage; Group II includes control rats given only adenine in a dose of 200 mg/kg three times a week orally for 8 weeks suspended in 10% Tween 80; Group III received ginger (500 mg/kg/day) orally 3 days before and three times weekly for 8 weeks concurrent with adenine; Group IV received AG (200 mg/kg) as ginger; Group V received Boswellia (900 mg/kg) as ginger; Group VI represents sham-operated rats; Group VII represents IR rats; Group VIII received ginger (500 mg/kg/day) orally 7 days before the induction of IR injury; Group IX received AG (200 mg/kg) orally 7 days before the induction of RIR injury; and Group X received Boswellia (900 mg/kg) orally 7 days before the induction of RIR injury.
Blood Sampling and Serum Preparation
At the end of the experiments, all animals from each group were anesthetized with urethane (1.3 g/kg). Blood sample of 3–5 mL was obtained from orbital sinus of rat using heparinized microcapillary tubes according to the method of Sorg and BucknerCitation12 and samples collected in clean dry test tubes were then centrifuged at 2000 × g for 15 min using Heraeus Sepatech centrifuge (Labofuge 200, Fischer Scientific, Pittsburgh, PA, USA). The serum was collected and divided into two aliquots: one used for the immediate determination of LDH and the second aliquot was kept at −20°C for further biochemical analysis.
Tissue Sampling
In CRF groups, animals were killed and dissected. One kidney from each rat was immediately removed and chilled in liquid nitrogen and kept at −20°C for SOD determination. The other kidney was also removed and kept in glutaraldehyde for electron microscopic examination. While in sham-operated and in IR groups, animals were killed and dissected and the remaining kidneys were immediately removed and chilled in liquid nitrogen and kept at −20°C for SOD determination.
Biochemical Analysis
Renal function tests
Serum creatinine was determined colorimetrically according to the method described by Bowers and Wong,Citation13 using a diagnostic kit supplied by the Egyptian Company for Biotechnology. Urea was determined by a colorimetric method according to the principle of Shephard and MezzachiCitation14 using a biodiagnostic kit supplied by the Egyptian Company of Biotechnology. Blood urea nitrogen (BUN) is determined by multiplying the result of serum urea by 0.467.
Cell death
Serum LDH as indicator of necrotic cell death was determined by a photometric method as described by VassaultCitation15 using a biodiagnostic kit supplied by Spinreact.
Lipid peroxidation
Serum MDA, a product of lipid peroxidation, was determined colorimetrically as described by SatohCitation16 using a diagnostic kit supplied by Bio Diagnostic Co.
Inflammation markers
Serum C-reactive protein (CRP), an indicator of inflammation, was determined by an enzyme immunoassay method as described by Helgeson et al.Citation17 using a diagnostic kit supplied by DiaMed EuroGen.
Antioxidant status
Kidney SOD was determined by a colorimetric method as described by Nishikimi et al.Citation18 using a diagnostic kit supplied by Bio Diagnostic Co.
Electron microscopic examination
One kidney from each rat of CRF groups was cut into longitudinal sections 2–4 mm in thickness and kept in glutaraldehyde. The kidneys were examined under electron microscope to determine ultrastructural changes and a photomicrograph was taken of one kidney from each group.
Statistical Analysis
All results are expressed as mean ± SEM. Statistical analysis was done using graph pad prism software version 5 (GraphPad Software, San Diego, CA, USA). The intergroup variation was measured by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc least significant difference test. A value of p < 0.05 was used as the limit for statistical significance.
RESULTS
Renal Function
shows that adenine administration induced a significant elevation of serum creatinine, urea, and BUN compared with normal rats (p < 0.05). It also increased the serum LDH levels (p < 0.05).
Table 1. Effect of oral administration 3 days before and 8 weeks concurrent with adenine (200 mg/kg) on renal function parameters and LDH activity in adult male albino rats.
Ginger and AG administration along with adenine reduced serum creatinine, urea, BUN, and normalized LDH levels (p < 0.05) when compared with adenine-treated rats. However, Boswellia reduced only serum urea and BUN levels when compared with adenine-treated rats.
shows that RIR injury also elevated serum creatinine, urea, and BUN compared with sham-operated rats (p < 0.05). It increased serum LDH levels (p < 0.05). Ginger, AG, and Boswellia administration 7 days before RIR reduced serum creatinine, urea, and BUN (p < 0.05) when compared with IR group. Only ginger and AG were able to reduce serum LDH levels.
Table 2. Effect of oral administration 7 days before 30 min ischemia and 24 h reperfusion (IR) on renal function parameters and LDH activity in adult male albino rats.
Oxidative Stress and Inflammation
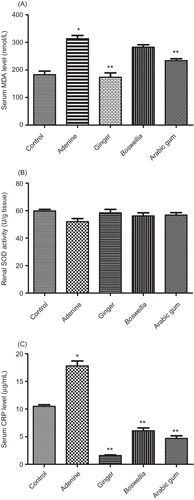
A shows that adenine caused a significant elevation in lipid peroxidation represented by increased MDA levels when compared with normal rats (p < 0.05). However, it did not affect renal SOD activity (B). Adenine elevated serum CRP level when compared with normal rats (C). Ginger and AG administration along with adenine reduced both serum MDA and CRP levels (A and C) when compared with adenine-treated rats (p < 0.05). Boswellia only reduced serum CRP levels when compared with adenine-treated rats (p < 0.05).
Figure 1. Effect of oral administration of Boswellia (900 mg/kg), ginger (500 mg/kg), and Arabic gum (200 mg/kg) 3 days before and 8 weeks concurrent with adenine (200 mg/kg) on serum MDA levels (A), renal SOD (B), and serum CRP levels (C) in adult male albino rats.
Notes: MDA, malondialdehyde; SOD, superoxide dismutase; CRP, C-reactive protein.
*Significantly different from control at p < 0.05; **significantly different from adenine group at p < 0.05.
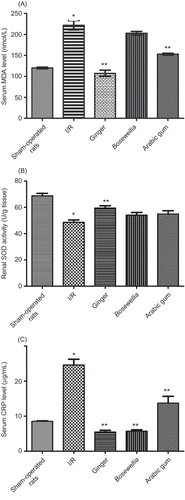
A and C shows that RIR caused significant elevation in lipid peroxidation (MDA levels) and serum CRP levels, respectively, when compared with sham-operated rats (p < 0.05). Renal SOD activity was reduced in IR group when compared with sham-operated rats (B). All drugs reduced serum CRP levels when compared with IR group (C). Ginger and AG reduced serum MDA levels (A). While ginger only was able to increase renal SOD activity. Boswellia had no significant effect on oxidative stress when compared with IR group (A and B).
Figure 2. Effect of oral administration of Boswellia (900mg/kg), ginger (500 mg/kg), and Arabic gum (200 mg/kg) for 7 days before RIR on serum MDA levels (A), renal SOD (B), and serum CRP levels (C) in adult male albino rats.
Notes: I/R, ischemia–reperfusion; MDA, malondialdehyde; SOD, superoxide dismutase; CRP, C-reactive protein.
*Significantly different from sham-operated rats at p < 0.05; **significantly different from ischemic–reperfusion (IR) group at p< 0.05.
Histopathological Findings
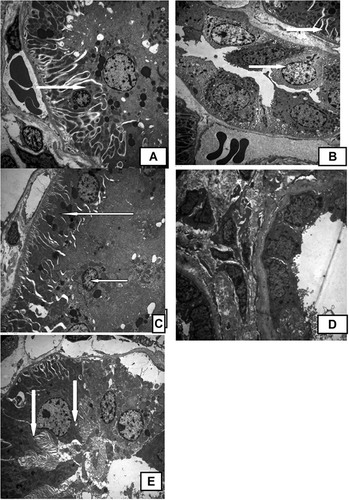
Rats in the control group showed normal kidney architecture and histology with normal ultrastructure of proximal tubules, cells with many electron-dense granules, apical vacuolations, and normal-shaped nuclei with apical microvilli and basal enfolding full of mitochondria (A). Adenine caused ultrastructural heterogeneity within proximal tubules and loss of microvilli. Some cells showed electron-dense cytoplasm and few mitochondria (B). Ginger-treated rats showed relatively normal proximal tubules. Blood capillaries had corrugated basement membrane and wide capillary spaces. There were electron-dense nuclei of blood vessels with irregular-shaped nuclei. There was an increase in mesangial cells. The podocytes showed dark cytoplasm with dark nuclei (C). Boswellia-treated rats showed ultrastructural damage within proximal tubules with very few electron-dense granules and irregular-shaped nuclei of some cells (D). AG caused an improvement of proximal tubules’ ultrastructural changes, wide intercellular spaces, corrugated nuclei, thickened basement membrane, and few electron-dense granules (E).
Figure 3. (A) Electron micrograph of normal rat kidney with normal ultrastructure of proximal tubules, cells with many electron-dense granules, apical vacuolations, and normal-shaped nuclei with apical microvilli and basal enfolding full of mitochondria (arrows); (B) electron micrograph of rat kidney treated with adenine with ultrastructural heterogeneity within proximal tubules, loss of microvilli, some cells show electron-dense cytoplasm, and few mitochondria (arrows); (C) electron micrograph of rat kidney treated with ginger with relatively normal proximal tubules, blood capillaries, corrugated basement membrane, wide capillary spaces, and electron-dense nuclei of blood vessels with irregular-shaped nuclei. There is increase in mesangial cells. The podocytes show dark cytoplasm with dark nuclei (arrows); (D) electron micrograph of rat kidney treated with Boswellia, showing ultrastructural damage within proximal tubules, very few electron-dense granules, and irregular-shaped nuclei of some cells; and (E) electron micrograph of rat kidney treated with AG, showing an improvement of proximal tubules, wide intercellular spaces, corrugated nuclei, thickened basement membrane, and few electron-dense granules (arrows).
Note: AG, Arabic gum.
DISCUSSION
This study was performed to demonstrate the role and different mechanisms of natural products such as ginger, AG, and Boswellia in repairing damaged kidney cells either in acute renal failure or CRF.
We showed that the oral administration of adenine caused renal dysfunction that is represented by an increase of serum creatinine, urea, and BUN levels. Electron microscopic examination of renal tubules revealed that adenine caused loss of microvilli of proximal tubule and the appearance of very low electron-dense cytoplasm with few mitochondria in some cells. This indicates a decrease in cellular surface area of the proximal tubule and destruction of sodium-potassium adenosine triphosphatase (Na+/K+–ATPase) pump due to low number of mitochondria. It was previously reported that adenine-treated kidneys were markedly enlarged with a pale gray color with the formation of foreign body granuloma in the tubular lamina and the interstitium of the kidney.Citation19
The model of adenine-induced CRF was developed by Yokozawa et al.,Citation10 in which long-term feeding of adenine to rats produced metabolic abnormalities resembling CRF in humans. In mammalian metabolism, when it is present in excess, adenine becomes a significant substrate for xanthine dehydrogenase, which can oxidize adenine to 2,8-dihydroxyadenine (DHA).Citation20 DHA is highly protein bound and is actively secreted by the renal tubules,Citation21 but it is sparingly soluble at the physiological urinary pH. The sparingly soluble nature of DHA results in the excretion of DHA crystals in urine and frequently, the deposition of stones in kidneys. Because adenine and DHA have very low solubilities, they precipitate in the tubules of the kidney.Citation22
The current study showed that adenine caused lipid peroxidation and inflammation, which ultimately caused renal cell death. This was confirmed by the significant increases in lipid peroxidation end product, MDA; acute phase inflammatory marker, CRP; and the elevation of serum LDH levels.
However, the activity of antioxidant enzyme SOD was not significantly changed by adenine in this study. A previous study found that the activity of SOD enzyme was significantly decreased in adenine-treated rats.Citation23 This difference in results may be attributed to the different period of adenine treatment where in the previous study adenine was given for only 4 weeks, but in our study adenine was given for 8 weeks. Kidneys may compensate for the increased oxidative stress by increasing the synthesis of SOD again to normal levels during the 8-week period.
In this study, oral administration of ginger along with adenine caused a significant improvement in renal function, represented by the significant decrease in serum creatinine, urea, and BUN. It also reduced serum LDH levels and attenuates ultrastructural changes. Electron microscopic examination showed relatively normal proximal tubules.
The mechanisms of these renoprotective effects may involve reduction of both oxidative stress and inflammation in renal cells. This is clear by significant reduction in lipid peroxidation (MDA) and serum CRP levels. It may be possible that 6-gingerol, one of the active constituents of ginger, due to its potential antioxidant properties,Citation24,25 improves renal functions by attenuating oxidative stress-mediated decline in glomerular filtration rate (GFR) and renal hemodynamics.
Ginger was found to scavenge hydroxyl, superoxide, and other free radicals in a dose-dependent manner in vitro.Citation26 In human aortic endothelial cells, zingerone and 6-gingerol demonstrated significant antioxidant effects on low-density lipoproteins.Citation27 Previous studies showed that ginger ameliorated cisplatin-induced nephrotoxicity either by preventing cisplatin-induced decline of renal antioxidant defense system or by its direct free radical scavenging activity.Citation28
It was also proved that ginger exhibits anti-inflammatory effects,Citation29 which give a good rationale for significant decrease in serum CRP levels observed in our study. Significant decreases in serum CRP levels are confirmed by recent studies in which [6]-gingerol is endowed with strong anti-inflammatory and antiapoptotic actions.Citation30
The results of this study illustrated that AG attenuated adenine-induced renal dysfunction which is proved by significant decrease in serum creatinine, urea, and BUN levels. Electron microscopic examination of renal tubules also showed an improvement of tubular epithelial cells. It has been previously reported that oral administration of AG had a protective action against gentamicin-induced nephrotoxicity,Citation31 while others reported only a modest nephroprotection by AG.Citation32
We showed that AG offered protection against lipid peroxidation, inflammation, and renal cell damage, which is revealed by decrease in serum MDA, CRP, and LDH levels.
The anti-inflammatory effect of AG is in accordance with Gamal El-din et al.,Citation33 who stated that AG has been reported to be used internally for the treatment of inflammation of the intestinal mucosa, and externally to cover inflamed surfaces.
It has also been postulated that AG enhances the amount of energy available to the colonies of bacteria that ferment dietary fibers and absorb nitrogen as they grow.Citation34 These bacterial colonies are also capable of degrading urea to ammonia, excreting it in feces, and taking some of the body nitrogen wastes with them.Citation8,9
The renoprotective effect of AG may be also attributed to the sorbent (binding) effect of AG that helps in removing some of the creatinine and urea from the blood without actually changing important physiological variables such as GFR and creatinine clearance. It is thought that oral sorbents correct malnutrition in CRF by binding with the toxins produced by uremia.Citation35 Collectively, the previous effects may be responsible for the reduction of renal cell death as indicated by the decrease of serum LDH levels shown in this study.
HuemerCitation36 suggested that natural substances which possess anti-inflammatory activity may help to stabilize failing kidneys. Among them are curcumin (from the spice turmeric), Boswellia herb (source of a natural 5-lipoxygenase inhibitor), and fish oils.
However, the results of this study showed that Boswellia caused a little improvement in kidney function. This is indicated by its ability to significantly reduce urea and BUN levels. But it had no significant effect on serum creatinine levels and it also did not reduce lipid peroxidation. It did not protect against renal tubular cell death as no change occurred in serum LDH levels.
Boswellia had only anti-inflammatory effect where it reduced serum CRP levels. These biochemical findings indicate that Boswellia has little beneficial effect in the protection against CRF. Electron microscopic examination of the kidney showed few electron-dense granules and irregular-shaped nuclei of some cells and no improvement in renal tubular cells. The anti-inflammatory effect of Boswellia may be due to 3-O-acetyl-11-keto-β-boswellic acid, which is the most active component of Boswellia extract and has been demonstrated to be a potent inhibitor of 5-Lipoxygenase (5-LOX), which is a key enzyme in the biosynthesis of leukotrienes (LTs) from arachidonic acid in the cellular inflammatory cascade.Citation37
The second model used in this work is RIR injury. It is a major cause of acute renal failure and renal graft rejection and may lead to CRF if chronic.Citation38,39 This investigation showed that rats subjected to 30-min ischemia showed a significant deterioration in renal function when measured 24 h after reperfusion as compared with respective sham-operated rats. This deterioration is indicated by significant increase in serum creatinine, urea, and BUN levels. RIR injury causes both renal and glomerular dysfunction Citation40 together with increased oxidative stress.Citation41,42
This study showed that RIR was associated with increased lipid peroxidation and reduction of renal SOD activity. In addition, it elevated serum CRP levels. Reactive oxygen species (ROS) such as hydrogen peroxide, superoxide, and hydroxyl radicals are generated in high concentration in ischemic tissue after reperfusion.Citation43 Although there is cellular defense against free radical injury provided by tissue SOD and free radical scavenging systems,Citation8 these systems are insufficient under certain conditions to prevent the damage totally.Citation44
Several studies have demonstrated that ROS have an important role in the RIR injury through lipid peroxidation of cells.Citation40 RIR injury in the kidney is associated with lipid peroxidation, which is an autocatalytic mechanism leading to oxidative destruction of cellular membranes.Citation42 This explains why RIR was associated in our study with elevation in serum LDH levels.
Another explanation of increased LDH levels is that proximal tubular cells with its highly selective transport mechanisms are severely disrupted due to the decline in ATP. This leads to dysfunction of the Na+/K+–ATPase pump located on the basolateral surface of proximal tubular cells that allows intracellular accumulation of Na+ ions followed by an influx of water leading to cell swelling, intracellular disruption, and eventual cell death.Citation41
Even when reperfusion of the kidney is established, additional RIR injury occurs. This involves the development of oxidative stress through the generation of superoxide anions O2•−, which has recently been measured as an indicator of RIR injury of the transplanted kidney.Citation45 Oxidative stress during RIR may elicit an inflammatory process observed in this study by the elevation of serum CRP levels. During reperfusion of ischemic tissue, an intense inflammatory response is induced.Citation46 This promotes the rapid infiltration of neutrophils, which are subsequently activated to dilute tissue damage during RIR.
The current study showed that oral administration of ginger for 7 days before RIR caused a significant improvement in all renal function parameters. This is indicated by the significant decrease in serum creatinine, urea, and BUN levels. The protective potential of ginger against RIR may be attributed to both antioxidant and anti-inflammatory effects.
This study showed that ginger treatment restored normal renal level of SOD enzyme and reduced lipid peroxidation. Previous studies showed that serum MDA is significantly reduced by ginger.Citation47
Ginger also reported to inhibit peroxidation of phospholipid liposomes in the presence of iron (III).Citation48 In rats, where free radicals were generated with organophosphate toxicity, supplementation with ginger provided a significant antioxidant effects, raising tissue concentrations of SOD, catalase, and reduced glutathione.Citation49 Ginger also reduced serum CRP levels in this work indicating potent anti-inflammatory effect.
This investigation showed that AG caused a significant improvement in kidney function by decreasing serum creatinine, urea, and BUN levels. It also has protective effect against cell damage caused by RIR, which is represented by significant decrease in serum LDH levels.
The mechanism of this protective effect may be due to antioxidant activities of AG which are supported by reduction in lipid peroxidation and elevation of renal SOD content. A series of articles showed a protective effect of AG against experimental GM and cisplatin nephrotoxicity,Citation32 doxorubicin cardiotoxicityCitation50 in rats, and acetaminophen hepatotoxicityCitation33 in mice. All these studies were based on the assumption that AG has strong antioxidant properties, and the major mechanism for the induction of these toxicities was the generation of free radicals.
In addition, dietary supplementation with AG, by increasing systemic levels of butyrate, may have a potential beneficial effect in renal disease by suppression of TGF-1 activity.Citation51
LTs are metabolites of arachidonic acid formed from the 5-LOX pathway and exert potent vasoactive and proinflammatory effects in conditions associated with RIR injury.Citation52 LTs also play a physiological role in the host defense against microbial infections. Thus, inhibitors of 5-LOX may be useful in the treatment of conditions associated with RIR injury.Citation53
In this work, we found that oral administration Boswellia carteri for 7 days before RIR process caused a significant improvement in kidney functions indicated by the reduction of serum creatinine, urea, and BUN levels.
This study showed that Boswellia exerted anti-inflammatory effect by decreasing serum CRP levels in RIR. This may give an explanation for the significant reduction of serum creatinine, urea, and BUN levels. Our results are matched with Patel et al.Citation54 who stated administration of zileuton (a potent 5-LOX inhibitor similar in mechanism of action to Boswellia) significantly attenuated renal dysfunction and injury caused by RIR of the mouse in vivo.
The reduction in serum CRP levels may be attributed to boswellic acids (BAs), which possess diverse pharmacological properties, including antiproliferative, proapoptotic and pro-differentiating, and anti-inflammatory effects, and are assumed as the active principles of Boswellia species extracts.Citation55
Accordingly, it is speculated that BAs may exert their anti-inflammatory effect mainly by inhibiting the release of proinflammatory LT products from leukocytes and plateletsCitation55 and by the inhibition of NF-κβ and subsequent downregulation of TNF- expression in activated monocytes.Citation56
The protective effect of Boswellia against RIR is not attributed to the modulation of oxidative stress as it does not affect serum MDA or renal SOD levels. But it may block its subsequent inflammatory effect.
It should be noted that the renal injury and dysfunction observed in rats treated with Boswellia were not entirely abolished. In addition, the degree of inhibition of serum urea and creatinine was not as complete as that of serum CRP. This is not surprising, given that many other pathophysiological mechanisms, which are independent of LTs and/or an enhanced inflammatory response, will contribute to the observed injury during ischemia and/or reperfusion. These mechanisms may include (but are not limited to) the generation of ROS and reactive nitrogen species, an enhanced formation of nitric oxide, modification of endogenous lipoxin generation, or the activation of the nuclear enzyme poly(ADP-ribose) polymerase.Citation57
In conclusion, it is clear that oxidative stress plays a crucial role in the development of adenine-induced renal failure and the more the potent antioxidant, the more the renoprotective effect. It is also clear that ginger is the most potent renoprotective agent against adenine-induced renal failure in this study followed by AG. However, Boswellia has the least protective effect.
This study also provided experimental evidence that ginger attenuated RIR-induced ARF, suggesting a promising potential of ginger in protecting against ARF. Ginger was more potent than AG in both models of renal failure. The mechanisms of nephroprotection of both ginger and AG may involve antioxidant and anti-inflammatory actions. It is also observed that inflammation has much greater role in RIR-induced renal dysfunction than in adenine-induced renal impairment, where Boswellia attenuates serum creatinine level in RIR but not in adenine-induced CRF. Further work on the effects of active constituents of ginger on both adenine-induced CRF and ARF is required to determine the most effective active constituent.
ACKNOWLEDGMENT
The authors thank Dr. Abier Azmy, assistant professor of histology, Faculty of Medicine, Zagazig University, for assistance in electron microscopic examination of kidney.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Lysaght MJ. Maintenance dialysis population dynamics: Current trends and long-term implications. J Am Soc Nephrol. 2002;13(1):37–40.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):204–212.
- Phillips S, Hutchinson S, Ruggier R. Zingiber officinale does not affect gastric emptying rate. A randomized, placebo-controlled, crossover trial. Anesthesia. 1993;48(5):393–395.
- Arfeen Z, Owen H, Plummer JL, Ilsley AH, Sorby-Adams RA, Doecke CJ. A double-blind randomized controlled trial of ginger for the prevention of postoperative nausea and vomiting. Anaesth Intensive Care. 1995;23(4):449–452.
- Grant KL, Lutz RB. Ginger. Am J Health Syst Pharm. 2005;57(10):945–947.
- Vutyavanich T, Kraisarin T, Ruangsri R. Ginger for nausea and vomiting in pregnancy: Randomized, double-masked, placebo-controlled trial. Obstet Gynecol. 2001;97(4):577–582.
- Fan AY, Lao L, Zhang RX, . Effects of an acetone extract of Boswellia carteri Birdw. (Burseraceae) gum resin on adjuvant-induced arthritis in Lewis rats. J Ethnopharmacol. 2005;101(1–3):104–109.
- Younes H, Garleb K, Behr S, Rémésy C, Demigné C. Fermentable fibers or oligosaccharides reduce urinary nitrogen excretion by increasing urea disposal in the rat cecum. J Nutr. 1995;125(4):1010–1016.
- Bliss DZ, Stein TP, Schleifer CR, Settle RG. Supplementation with gum Arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr. 1996;63(3):392–398.
- Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron. 1986;44(3):230–234.
- Rabb H, Mendiola CC, Saba SR, . Antibodies to ICAM-1 protect kidneys in severe ischemic reperfusion injury. Biochem Biophys Res Commun. 1995;211(1):67–73.
- Sorg DA, Buckner B. A simple method of obtaining venous blood from small laboratory animals. Proc Soc Exp Biol Med. 1964;115:1131–1132.
- Bowers LD, Wong ET. Kinetic serum creatinine assays. II. A critical evaluation and review. Clin Chem. 1980;26(5):555–561.
- Shephard MD, Mezzachi RD. Scientific and Technical Committee: Technical Report No. 8. The collection, preservation, storage and stability of urine specimens for routine clinical biochemical analysis. Clin Biochem Rev.1983;4:61–67.
- Vassault A. Lactate dehydrogenase. UV-method with pyruvate and NADH. In: Bergmeyer J, Grabl M, eds. Methods of Enzymatic Analysis. Deerfield Beach, FL: Verlag-Chemie; 1983:119–126.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1987;90(1):37–43.
- Helgeson NGP, Adamson DM, Pike RB, . C-reactive protein. In: Race GJ, ed. Laboratory Medicine, Vol. 2, Chap. 29. Hagerstown: Harper & Row; 1973.
- Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46(2):849–854.
- Yokozawa T, Oura H, Nakagawa H, Okada T. Adenine induced hyperuricemia and renal damage in rats. Nippon Nogeikagaku Kaishi. 1982;56:655.
- Stockelman MG, Lorenz JN, Smith FN, . Chronic renal failure in a mouse model of human adenine phosphoribosyltransferase deficiency. Am J Physiol. 1998;275(1):154–163.
- Ericson A, Groth T, Niklasson F, de Verdier CH. Plasma concentration and renal excretion of adenine and 2, 8-dihydroxyadenine after administration of adenine in man. Scand J Clin Lab Invest. 1980;40(1):1–8.
- Peck CC, Baily FJ, Moore GL. Enhanced solubility of 2,8-dihydroxyadenine (DOA) in human urine. Transfusion. 1977;17:383–390.
- Ali BH, Al-Salam S, Al Husseni I, . Effects of Gum Arabic in rats with adenine-induced chronic renal failure. Exp Biol Med. 2010;235:373–382.
- Afzal M, Al-Hadidi D, Menon M, Pesek J, Dhami MS. Ginger: An ethnomedical, chemical and pharmacological review. Drug Metabol Drug Interact. 2001;18(3–4):159–190.
- Shukla Y, Singh M. Cancer preventive properties of ginger: A brief review. Food Chem Toxicol. 2007;45(5):683–690.
- Jagetia G, Baliga M, Venkatesh P. Ginger (Zingiber officinale Rosc.), a dietary supplement, protects mice against radiation-induced lethality: Mechanism of action. Cancer Biother Radiopharm. 2004;19 (4):422–435.
- Fuhrman B, Rosenblat M, Hayek T, Coleman R, Aviram M. Ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. J Nutr. 2000;130(5):1124–1131.
- Ajith TA, Nivitha V, Usha S. Zingiber officinale Roscoe alone and in combination with alpha-tocopherol protect the kidney against cisplatin-induced acute renal failure. Food Chem Toxicol. 2007;45(6):921–927.
- Young HY, Luo YL, Cheng HY, Hsieh WC, Liao JC, Peng WH. Analgesic and anti-inflammatory activities of [6]-gingerol. J Ethnopharmacol. 2005;96(1–2):207–210.
- Kim JK, Kim Y, Na KM, Surh YJ, Kim TY. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic Res. 2007;41(5):603–614.
- Gilbert DN, Wood CA, Kohlhepp SJ, . Polyaspartic acid prevents experimental aminoglycoside nephrotoxicity. J Infect Dis. 1989;159(5):945–953.
- Al-Majed AA, Mostafa AM, Al-Rikabi AC, Al-Shabanah OA. Protective effects of oral Arabic gum administration on gentamicin-induced nephrotoxicity in rats. Pharmacol Res. 2002;46(5):445–451.
- Gamal El-din AM, Mostafa AM, Al-Shabanah OA, Al-Bekairi AM, Nagi MN. Protective effect of Arabic gum against acetaminophen-induced hepatotoxicity in mice. Pharmacol Res. 2003;48(6):631–635.
- Ali BH, Al-Qarawi AA, Haroun EM, Mousa HM. The effect of treatment with gum Arabic on gentamicin nephrotoxicity in rats: A preliminary study. Ren Fail. 2003;25(1):15–20.
- Tsubakihara Y, Takabatake Y, Oka K, . Effects of the oral adsorbent AST-120 on tryptophan metabolism in uremic patients. Am J Kidney Dis. 2003;41(3 Suppl. 1):S38–S41.
- Huemer RP. Chronic renal disease: Orthomolecular ramifications. J Orthomolecular Med. 2006;21(1):48–54.
- Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon HP, Safayhi H. Acetyl-11-keto-beta-boswellic acid (AKBA): Structure requirements for binding and 5-lipoxygenase inhibitory activity. Br J Pharmacol. 1996;117(4):615–618.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334(22):1448–1460.
- Gilbert RE, Kelly DJ, Atkins RC. Novel approaches to the treatment of progressive renal disease. Curr Opin Pharmacol. 2001;1(2):183–189.
- Paller MS. The cell biology of reperfusion injury in the kidney. J Investig Med. 1994;42(4):632–639.
- Sheridan AM, Bonventre JV. Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr Opin Nephrol Hypertens. 2000;9(4):427–434.
- Eschwège P, Paradis V, Conti M, . In situ detection of lipid peroxidation by-products as markers of renal ischemia injuries in rat kidneys. J Urol. 1999;162(2):553–557.
- Haugen E, Nath KA. The involvement of oxidative stress in the progression of renal injury. Blood Purif. 1999;17(2–3):58–65.
- Halliwell B, Cross CE. Oxygen-derived species: Their relation to human disease and environmental stress. Environ Health Perspect. 1994;102(10):5–12.
- Masztalerz M, Włodarczyk Z, Czuczejko J, Słupski M, Kedziora J. Superoxide anion as a marker of ischemia-reperfusion injury of the transplanted kidney. Transplant Proc. 2006;38(1):46–48.
- Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123(1):7–13.
- Uz E, Karatas OF, Mete E, . The effect of dietary ginger (Zingiber officinals Rosc) on renal ischemia/reperfusion injury in rat kidneys. Ren Fail. 2009;31(4):251–260.
- Aeschbach R, Löliger J, Scott BC, . Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol. 1994;32(1):31–36.
- Ahmed RS, Seth V, Pasha ST, Banerjee BD. Influence of dietary ginger (Zingiber officinale Rosc) on oxidative stress induced by malathion in rats. Food Chem Toxicol. 2000;38(5):443–450.
- Abd-Allah AR, Al-Majed AA, Mostafa AM, Al-Shabanah OA, Din AG, Nagi MN. Protective effect of Arabic gum against cardiotoxicity induced by doxorubicin in mice: A possible mechanism of protection. J Biochem Mol Toxicol. 2002;16(5):254–259.
- Matsumoto N, Riley S, Fraser D, . Butyrate modulates TGF-beta1 generation and function: Potential renal benefit for Acacia (sen) SUPERGUM (gum Arabic)? Kidney Int. 2006;69(2):257–265.
- Carter GW, Young PR, Albert DH, . 5-Lipoxygenase inhibitory activity of zileuton J Pharmacol Exp Ther. 1991;256(3):929–937.
- Demitsu T, Katayama H, Saito-Taki T, Yaoita H, Nakano M. Phagocytosis and bactericidal action of mouse peritoneal macrophages treated with leukotriene B4. Int J Immunopharmacol. 1989;11(7):801–808.
- Patel NS, Collin M, Thiemermann C. Urocortin does not reduce the renal injury and dysfunction caused by experimental ischemia/reperfusion. Eur J Pharmacol. 2004;496(1–3):175–180.
- Poeckel D, Tausch L, George S, Jauch J, Werz O. 3-O-acetyl-11-keto-boswellic acid decreases basal intracellular Ca2+ levels and inhibits agonist-induced Ca2+ mobilization and mitogen-activated protein kinase activation in human monocytic cells. J Pharmacol Exp Ther. 2006;316:224–232.
- Syrovets T, Büchele B, Krauss C, Laumonnier Y, Simmet T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. J Immunol. 2005;174(1):498–506.
- Chatterjee PK, Zacharowski K, Cuzzocrea S, Otto M, Thiemermann C. Inhibitors of poly (ADP-ribose) synthetase reduce renal ischemia-reperfusion injury in the anesthetized rat in vivo. FASEB J. 2000;14:641–651.
