Abstract
Background: There are few data on the effects of anesthesia and cardiopulmonary bypass (CPB) on perioperative renal function in children with cyanotic congenital heart disease undergoing open heart surgery. This study aims to investigate the perioperative renal function in cyanotic versus acyanotic children undergoing sevoflurane anesthesia for open heart surgery. Methods: After receiving ethical committee approval, 12 acyanotic patients (preoperative oxygen saturation: SaO2 > 85%) and 12 cyanotic children (SaO2 < 85%) were included. Sevoflurane was administered at concentration levels of 2% before CPB and 1–2% during CPB after standard anesthesia induction. Inorganic fluoride, electrolytes, creatinine, urea nitrogen in serum and urine samples, and N-acetyl-β-d-glucosaminidase (NAG) in urine samples were measured before induction, before CPB, during CPB, after CPB, at the end of surgery, and at 24th h postoperatively. Results: The levels of serum uric acid levels were higher in the cyanotic group (p < 0.05). There were no differences in the levels of serum creatinine and urine creatinine, urea nitrogen, and electrolytes between the two groups. Serum inorganic fluoride levels were always higher in the acyanotic group than in the cyanotic group, but these differences between the groups reached statistical significance at two measurement times (before CPB and end of surgery) (p < 0.05). Urinary inorganic fluoride levels increased with time in both groups. Although urinary NAG increased significantly after the CPB in the cyanotic group, the differences between the two groups did not reach statistical significance. Conclusions: We have concluded that renal function was not affected during open heart surgery with sevoflurane anesthesia, in both cyanotic and acyanotic children.
INTRODUCTION
Children with complex acyanotic and cyanotic heart disease undergoing cardiopulmonary bypass (CPB) are at risk for postoperative renal impairment.Citation1 The reported incidence of acute kidney injury is approximately 5–20% and is related to the complexity of the cardiac surgery, hemodynamic alterations, and duration of CPB time.Citation2,3 CPB triggers an important inflammatory reaction and may lead to hemodynamic alterations and renal hypoperfusion.Citation4 Renal hypoperfusion during CPB may be especially deleterious in cyanotic children with already decreased renal plasma flow.Citation1,5–7 Thus, assessing alterations of renal function integrity in patients undergoing cardiac operation appears to be of high importance in developing strategies to avoid renal dysfunction in the postoperative period.Citation8
It is important to avoid potentially nephrotoxic substances when performing anesthesia in these children with high risk for renal dysfunction. Currently, sevoflurane, a potent volatile anesthetic agent, is widely used for induction and maintenance of anesthesia in pediatric cardiac surgery. Although no renal impairment has been documented in healthy children, sevoflurane should be used with caution in patients with renal impairment due to concerns over the potential nephrotoxicity of the sevoflurane by-products: pentafluoroisopropenyl fluoromethyl ether (compound A) and plasma fluoride ions.Citation9,10
This study was designed to compare the perioperative renal functions in cyanotic and acyanotic children undergoing sevoflurane anesthesia during open heart surgery. Renal function was assessed by clinical laboratory markers of kidney function, electrolytes, and by a subclinical, sensitive marker of tubular damage [urinary N-acetyl-β-d-glucosaminidase (NAG)].
METHODS
Clinical Protocol
After ethics committee approval and parental consent, 59 patients 1–6 years of age with cyanotic and acyanotic congenital cardiac anomalies scheduled for cardiac surgery at the Hacettepe University Hospital were included in this study. Patients with renal or hepatic failure, diabetes mellitus, heart failure, acute or chronic pulmonary disease, pulmonary artery hypertension, previous cardiac surgery, or anomalies of other organ systems were excluded. Patients requiring surgical revision for any reason; patients receiving mechanical ventilation for more than 24 h and those who required peritoneal dialysis, hemodialysis, or ultrafiltration intraoperatively or postoperatively; and patients with preoperative or postoperative inotropic support and those who died in an early period were also excluded from this study.
The patients were divided into two groups according to the oxygen saturation measured by pulse oximetry: the acyanotic group (O2 sat ≥ 85%) and the cyanotic group (O2 sat < 85%). Patients were premedicated with midazolam (0.5 mg/kg intranasal) 30 min before surgery.
Anesthesia was induced with thiopental sodium 5 mg/kg, morphine HCl 0.1 mg/kg, and vecuronium bromide 0.1 mg/kg and maintained with morphine, vecuronium, and sevoflurane 2% together with 3 L/min nitrous oxide and 3 L/min oxygen using a semiclosed circle system with a soda lime canister before CPB and sevoflurane 1–2% during CPB. The depth of anesthesia was monitored with bispectral index (BIS™) (Aspect Medical Systems, Newton, MA, USA), and acceptable anesthetic depth (between 30 and 50) was achieved.
Heparin sulfate (300 U/kg) was given prior to cannulation. Activated clotting time (Hemochron 80, International Technidyne Corp., Edison, NJ, USA) was kept at ≥400 s during extracorporeal circulation. At the end of CPB circulating heparin was antagonized by protamine (3 mg/kg), and additional doses of protamine were given if needed.
DeBakey roller pump, membrane oxygenator [Dideco Membrane 901, 902, or 705 (Sorin Group, Mirandola, Italy) according to the weight of the patient], nonpulsatile flow (200 mL/kg/min), and mild hypothermia (26–28°C) were used for CPB. Priming fluid of the CPB pump consisted of fresh packed red blood cells, fresh frozen plasma, and Isolyte S solution. Standard cold cardioplegia was used at a dose of 15 mL/kg. The hematocrit level in CPB was kept ≥30% for patients weighing ≥20 kg or ≥24% for patients weighing <20 kg. Mannitol 20%, 1 g/kg, was also added to the priming volume.
All operations were performed by the same anesthesia and surgical team. Aortic cross-clamping time, CPB time, duration of operation and anesthesia, and data regarding hemodynamic parameters, urine output, blood and blood product requirements, and total amount of drainage were recorded.
The pH was kept within normal range. At the end of surgery, patients were transported to the intensive care unit. All patients received mechanical ventilation for at least 6 h. Data regarding urine output were recorded.
First blood samples were collected before anesthesia induction. First urine samples were collected after urinary catheter was placed just after induction. Further blood and urine samples were collected before onset of CPB (5 min after heparin administration), at 15th min of CPB, after CPB (5 min after protamine administration), at the end of surgery, and 24 h after surgery. Sodium (Na+), potassium (K+), calcium (Ca++), chloride (Cl−), creatinine (Cr), urea nitrogen, uric acid, and inorganic fluoride were measured in blood samples and urine specimen using standard laboratory techniques. Urinary NAG concentrations were determined by spectrophotometric assay using sodium cresolsulphonphtalein NAG (HITACHI 7150 type automatic analyzer; Hitachi Co., Tokyo, Japan).Citation5 Plasma inorganic fluoride concentrations were analyzed using an ion-selective electrode.Citation6 The potential values of the solutions were measured using ORION Model 720-A mV/pH meter (Thermo Electron Co., New York, USA) with ORION 9409 BN fluoride electrode (Thermo Electron Co.) and ORION Double Junction reference electrode (Ag–AgCl) (Thermo Electron Co.). Values less than 0.05 μmol/L could not be measured.
Statistical Analysis
All data were expressed as mean and standard deviation (mean ± SD) or median (95% confidence intervals) unless otherwise indicated. All categorical variables were tested by chi-square test. All normally distributed data (tested by Kolmogorov–Smirnov test) were analyzed using the unpaired t-test. One-way and two-way analysis of variance with repeated measures and post hoc Scheffe test were used to determine the effect of group, time, and group–time interaction. The Mann–Whitney U-test or Kruskal–Wallis test and Friedman tests were used when appropriate (for the analysis of non-normal distribution data). Pearson correlation analysis was used for the determination of correlation coefficients. A p-value less than 0.05 was considered significant. All data were entered and processed by SPSS 11.0 (SPSS Inc., Chicago, IL, USA) for Windows statistical package.
RESULTS
There were no differences between the groups regarding gender, age, body weight, duration of operation, aortic cross-clamping time, CPB time, duration of the anesthesia, and surgery (). Preoperatively, the cyanotic patients had lower oxygen saturation and higher hemoglobin and hematocrit values than the acyanotic patients (p < 0.05). Four patients of the acyanotic group had atrial septal defect, whereas eight had ventricular septal defect. In the cyanotic group, all patients had Tetralogy of Fallot. A total of 42 cyanotic and 17 acyanotic patients were eligible for the study. Three cyanotic patients and 1 acyanotic patient underwent revision for bleeding, 6 cyanotic and 2 acyanotic children required ventilatory support more than 24 h, 7 cyanotic and 3 acyanotic patients required dialysis, 30 cyanotic and 5 acyanotic patients required inotropic support and were excluded from the study. After those exclusions, the study was continued with 12 cyanotic and 12 acyanotic patients. They all underwent total corrective interventions. No diuretic other than mannitol was used in the bypass priming solution.
Table 1. Comparison of acyanotic and cyanotic groups (values are mean ± SD).
There were no differences in plasma Na+, K+, Ca++, and Cl− levels between the two groups (). In both groups, serum electrolytes showed generally similar trends; the levels gradually decreased starting before the onset of CPB, during CPB, until the end of CPB, and gradually returned to preinduction levels.
Table 2. Serum biochemistry of cyanotic and acyanotic groups.
There were no differences in serum Cr and blood urea nitrogen (BUN) levels between the two groups (). The serum uric acid levels were significantly higher in the cyanotic group than in the acyanotic group (p < 0.05).
Urinary Na+, K+, Cl−, Cr, and uric acid levels did not differ between the groups (). Urinary Ca++ level was lower in the acyanotic group than in the cyanotic group, 24 h after surgery (p < 0.05). Urinary urea nitrogen levels were higher in the acyanotic group than in the cyanotic group before induction and before the onset of CPB, but the urea nitrogen levels were similar between the two groups later on (p < 0.05). The urine output was higher in the acyanotic group than in the cyanotic group during CPB (p < 0.05).
Table 3. Urine volumes and biochemistry of cyanotic and acyanotic groups.
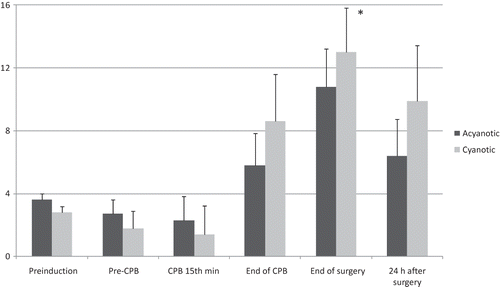
Serum inorganic fluoride levels were always higher in the acyanotic group than in the cyanotic group, but these differences in between the groups reached statistical significance only at two measurement times (pre-CPB and at the end of surgery) () (p < 0.05). Urinary inorganic fluoride levels increased in time in both groups, but the measured values were not statistically different between the groups ().
Figure 1. Serum inorganic fluoride levels (μmol/L).
Notes: Values are mean ± SD. **p < 0.05, when compared with preinduction values in the same group; *p < 0.05, between the two groups.
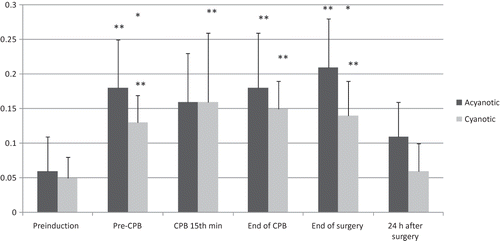
Figure 2. Urine inorganic fluoride levels (μmol/L).
Notes: Values are mean ± SD. *p < 0.05, when compared with the preinduction values in the same group.
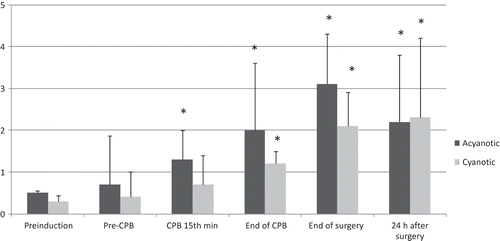
In the cyanotic group, urine NAG excretion was increased at the end of surgery when compared with the levels before the induction (). Although urinary NAG increased significantly after the CPB in the cyanotic group, the differences between the two groups did not reach statistical significance.
DISCUSSION
We were interested in the clinical and subclinical renal function changes in routine pediatric cardiac cases exposed to sevoflurane anesthesia. We compared cyanotic patients with acyanotic patients. The two groups were similar regarding serum and urine biochemistry from the beginning of anesthesia until the 24 h after surgery. The two groups did not differ regarding clinical and subclinical renal function tests on the day after the operation.
Nephropathy is recognized as a potential complication of cyanotic congenital heart disease, and the risk rises sharply during the second decade of life.Citation7 Glomerular and tubular dysfunction in longstanding cyanotic heart disease is related to the duration and degree of cyanosis and the extent to which the hematocrit is elevated.Citation7,11 Our cyanotic group consisted of young patients (mean age 40.3 months) so we did not detect any baseline overt clinical renal insufficiency. The deranged intrarenal hemodynamics in patients with cyanotic heart disease are associated with proteinuria, increased renal blood flow, increased renal vascular resistance, decreased renal plasma flow, normal or reduced glomerular filtration rates, and histologic changes on biopsy or at autopsy.Citation5,12 Cyanotic glomerulopathy has been shown to be associated with elevated hematocrit. Perfusion of the tubular apparatus, which is most vulnerable to ischemic damage, is provided by the capillaries of the peritubular plexus. To maintain normal blood flow in the peritubular capillaries, as there is increased resistance to flow of viscous blood in patients with elevated hematocrit, a high intravascular pressure is needed, which makes these patients easily susceptible to renal injury during CPB.Citation1
We measured NAG as a sensitive marker of proximal lysosomal tubular damage because standard markers of renal damage such as creatinine and creatinine clearance are not sensitive enough to detect discrete changes in renal function.Citation8 The transient increase observed in NAG excretion at the end of surgery was indicative of some mild tubular renal damage. Despite these data, the urine output and other biochemical markers remained within normal limits after surgery with a declining trend in electrolyte levels from the baseline, which may be due to the dilution as a result of fluid replacement and the priming solution of the CPB. In another study in 34 patients weighting less than 10 kg, repeated analysis of urine, blood, and plasma viscosity were performed only during CPB.Citation13 Polyuria and proteinuria that appeared during CPB indicated an elevated transglomerular filtration gradient, which recovered within 24 h. Similar to our results, the appearance of NAG in the urine was additionally indicative of mild tubular damage.Citation13 The increase in urinary NAG excretion was not indicative of clinically significant renal damage in our patients.
The plasma creatinine concentration has been validated as clinically important for renal function.Citation14 However, the urinary creatinine concentrations showed similar trends in both groups; the urinary creatinine levels decreased during and after CPB compared with the baseline. Although plasma urea concentration is less reliable than creatinine, along with creatinine, urea is frequently used in routine chemistry.Citation14 The blood and urine nitrogen concentrations were similar between the two groups, and this finding associated with sevoflurane anesthesia also accords with the findings associated with halothane anesthesia.Citation15
Acute changes in hematocrit affect renal plasma flow and renal vascular resistance. Palliative surgery has a significant improving effect on hematocrit and oxygen saturation level as well as renal glomerular and tubular integrity.Citation11 We believe that the beneficial effect of corrective surgery in cyanotic patients reverses the detrimental effects of CPB on renal function. This may explain the improvement of the perioperative renal dysfunction on the first postoperative day.
Reduced uric acid clearances in infants, young children with congenital heart disease, and in late survivors with cyanotic congenital heart disease have been reported to be secondary to inappropriately low fractional uric acid excretion.Citation12,16 In our study, urinary uric acid excretions were also lower in the cyanotic patients than in the acyanotic patients, although these differences did not reach statistical significance. Enhanced urate reabsorption appears to result from renal hypoperfusion reinforced by high filtration fraction.Citation12 The serum uric acid levels have been found previously to be higher in the cyanotic children compared with the acyanotic children undergoing cardiac operations.Citation17 Hyperuricemia serves as a marker of abnormal intrarenal hemodynamics.Citation12 In our patient cohort, serum uric acid levels at all times were higher in the cyanotic patients compared with the acyanotic patients. Classifications such as acute kidney injury network (AKIN) and risk, injury, failure, loss, end-stage (RIFLE) are used for evaluating the severity of postoperative renal dysfunction in adults.Citation18 Although pediatric RIFLE (pRIFLE) classification has been proposed for children, it has not been used for patients undergoing congenital cardiac surgery.Citation19 Therefore, we did not use pRIFLE or AKIN for assessment.
Numerous factors have been postulated to cause renal dysfunction after anesthesia and cardiac surgery. Besides the anesthesia technique, these factors include but not limited to antibiotics, surgical stress, preoperative renal dysfunction, intraoperative hemodynamic, use of dopamine, diuretics, type of the surgery, and patient positioning.Citation2,20,21 In our study, we tried to standardize the prophylactic antibiotics, intraoperative hemodynamic, CBP method, type, and dosage of anesthetics in all patients.
The introduction of sevoflurane into clinical anesthesia has been clouded by concerns about the potential risk for nephrotoxicity after its use. Theoretical sources for the nephrotoxicity would be hemodynamic instability and the metabolites of sevoflurane (fluoride and compound A). Most of the in vivo and in vitro studies revealed no major harmful effects of sevoflurane anesthesia on kidneys. Lee et al.Citation22 demonstrated the anti-inflammatory and anti-necrotic effects of sevoflurane in cultured kidney proximal tubule cells and probed the mechanism of sevoflurane-induced renal cell protection. Similarly, Şekeroğlu et al.Citation23 found out that although there is an acute altering in renal glomerular function with sevoflurane, this does not have a significant effect on biochemical markers of renal tubular damage. In another randomized study comparing sevoflurane and halothane anesthesia in infants and children with congenital heart disease, sevoflurane had hemodynamic advantages over halothane.Citation24 The plasma concentration of inorganic fluoride, an in vivo metabolite of sevoflurane, was found to be low [less than 50 μmol/L (1.02 ppm) theoretical threshold for nephrotoxicity] in children after sevoflurane anesthesia and was eliminated rapidly, and the children scheduled for elective surgery were unlikely to be at risk for nephrotoxicity from high fluoride levels.Citation25 In our study, the serum inorganic fluoride levels were also low (less than the theoretical threshold for nephrotoxicity). Similar to our results, the serum inorganic fluoride levels were even lower in the cyanotic group than in the acyanotic group. The plasma fluoride concentrations were consistent with other reported values in children.Citation26 The urinary fluoride levels were always higher in the acyanotic group than in the cyanotic group until the end of the operation, although these differences between the two groups did not reach statistical significance. It is difficult to explain the relatively higher fluoride levels and fluoride excretion in the acyanotic children. The concentration of compound A, an in vitro metabolite of sevoflurane, during sevoflurane anesthesia, using approximately 2 L fresh gas flow in a circle absorption system, has been found to be low in pediatric patients.Citation26 Although we did not measure compound A levels, we think our patients were exposed to even lower compound A levels because we used high fresh gas flow rates (6 L/min) and a semiclosed circle system. Exclusion of children with congenital heart disease at high risk for acute renal injury may have also affected our results.
The relatively small number of patients and younger patient age in the cyanotic group are limitations of this study. According to the observed data, we have concluded that sevoflurane anesthesia and CBP do not adversely affect renal functions in both cyanotic and acyanotic children undergoing open heart surgery. Further studies should also include children with high risk for acute renal failure.
ACKNOWLEDGMENT
This study was financially supported by the Department of Anesthesiology and Reanimation, Hacettepe University Faculty of Medicine.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Dittrich S, Kurschat K, Dahnert I, . Renal function after cardiopulmonary bypass surgery in cyanotic congenital heart disease. Int J Cardiol. 2000;73:173–179.
- Picca S, Principato F, Mazzera E, . Risks of acute renal failure after cardiopulmonary bypass surgery in children: A retrospective 10-year case-control study. Nephrol Dial Transplant. 1995;10:630–636.
- Owens GE, King K, Gurney JG, Charpie JR. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol. 2011;32:183–188.
- Kist-van Holthe tot Echten JE, Goedvolk CA, Doornaar MB, . Acute renal insufficiency and renal replacement therapy after pediatric cardiopulmonary bypass surgery. Pediatr Cardiol. 2001;22:321–326.
- Burlet A, Drukker A, Guignard JP. Renal function in cyanotic congenital heart disease. Nephron. 1999;81:296–300.
- Lazenby WD, Ko W, Zelano JA, . Effects of temperature and flow rate on regional blood flow and metabolism during cardiopulmonary bypass. Ann Thorac Surg. 1992;53:957–964.
- Dittrich S, Haas NA, Bührer C, Muller C, Dähnert I, Lange PE. Renal impairment in patients with long-standing cyanotic congenital heart disease. Acta Paediatr. 1998;87:949–954.
- Hauer D, Kilger E, Kaufmann I, . Risk and outcome analysis of renal replacement therapies in patients after cardiac surgery with pre-operatively normal renal function. Anaesthesia. 2009;64:615–619.
- Muller CM, Krenn CG, Urak G, Zimpfer M, Semsroth M. Sevoflurane in pediatric anesthesia. Acta Anaesthesiol Scand Suppl. 1997;111:150–151.
- Goa KL, Noble S, Spencer CM. Sevoflurane in paediatric anesthesia: A review. Paediatr Drugs. 1999;1:127–153.
- Awad H, el-Safty I, Abdel-Gawad M, el-Said S. Glomerular and tubular dysfunction in children with congenital cyanotic heart disease: Effect of palliative surgery. Am J Med Sci. 2003;325:110–114.
- Ross EA, Perloff JK, Danovitch GM, Child JS, Canobbio MM. Renal function and urate metabolism in late survivors with cyanotic congenital heart disease. Circulation. 1986;73:396–400.
- Dittrich S, Priesemann M, Fischer T, . Hemorheology and renal function during cardiopulmonary bypass in infants. Cardiol Young. 2001;11:491–497.
- Story DA, Poustie S, Liu G, McNicol PL. Changes in plasma creatinine concentration after cardiac anesthesia with isoflurane, propofol, or sevoflurane: A randomized clinical trial. Anesthesiology. 2001;95:842–848.
- Moore RA, McNicholas KW, Gallagher JD, . Halothane metabolism in acyanotic and cyanotic patients undergoing open heart surgery. Anesth Analg. 1986;65:1257–1262.
- Mace SE, Newman AJ, Liebman J. Impairment of urate excretion in patients with cardiac disease. Am J Dis Child. 1984;138:1067–1070.
- Ellis EN, Brouhard BH, Conti VR. Renal function in children undergoing cardiac operations. Ann Thorac Surg. 1983;36:167–172.
- Englberger L, Suri RM, Li Z, . Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15:R16.
- Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38:933–939.
- Ebert TJ, Arain SR. Renal responses to low-flow desflurane, sevoflurane, and propofol in patients. Anesthesiology. 2000;93:1401–1406.
- Kanbak M. Phenylephrine, dopamine, mannitol and renal protection during cardiopulmonary bypass. Anesth Analg. 1998;87:1458–1459.
- Lee HT, Kim M, Jan M, Emala CW. Anti-inflammatory and anti-necrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol. 2006;291:F67–F78.
- Şekeroğlu MR, Katı İ, Noyan T, Dülger H, Yalçınkaya AS. Alterations in the biochemical markers of renal function after sevoflurane anaesthesia. Nephrology 2005;10:544–547.
- Russell IA, Miller Hance WC, Gregory G, . The safety and efficacy of sevoflurane anesthesia in infants and children with congenital heart disease. Anesth Analg. 2001;92:1152–1158.
- Levine MF, Sarner J, Lerman J, . Plasma inorganic fluoride concentrations after sevoflurane anesthesia in children. Anesthesiology. 1996;84:348–353.
- Frink EJ Jr, Green WB Jr, Brown EA, . Compound A concentrations during sevoflurane anesthesia in children. Anesthesiology. 1996;84:566–571.