Abstract
Objective: To explore the association of urinary podocyte excretion and renal expression of podocyte-specific marker podocalyxin (PCX) with clinicopathological changes in immunoglobulin A nephropathy (IgAN). Methods: Morning urine samples from IgAN patients and healthy controls were collected. The expression of glomerular PCX was quantified in 50 IgAN patients diagnosed by renal biopsy. IgAN was classified based on the Lee’s Grading system and scored according to the Katafuchi semiquantitative criteria. Morphological evaluation of podocyte was determined by electron microscopy. Results: The amount of urinary podocytes in the IgAN patients was significantly higher than that in the healthy controls (p < 0.01). Pairwise comparison among Lee’s grades of IgAN showed that the median of urinary podocytes in Lee’s I–II group was lower than that in Lee’s III, IV, and V groups (p < 0.05); group III lower than group V (p < 0.05). The positive rate of urinary podocytes was the highest in Lee’s IV and V groups (100%), and lowest in Lee’s I–II group (55%). Multiple comparison among groups of Lee’s grades of IgAN showed that the glomerular PCX expression in Lee’s I–II group was higher than that in Lee’s III, IV, and V groups (p < 0.05); groups III and IV higher than group V (p < 0.05). The amount of urinary podocytes in IgAN patients was negatively correlated with PCX expression (r = −0.702, p < 0.01), but positively correlated with 24-h urinary protein (r = 0.465, p < 0.01) and glomerular (r = 0.233, p < 0.01) and renal tubular pathological scores (r = 0.307, p < 0.05). The glomerular PCX expression was negatively correlated with 24-h urinary protein (r = −0.367, p < 0.05) and glomerular (r = −0.560, p < 0.05) and tubular pathological scores (r = −0.377, p < 0.05). Electron microscopy showed significant changes in podocytes of IgAN, especially in the foot process. Conclusion: The amount of urinary podocyte can reflect the loss of podocytes in renal tissue, which may be a marker of IgAN progression.
INTRODUCTION
Immunoglobulin A nephropathy (IgAN) is one of the most common glomerular diseases. It is characterized by the predominate deposition of IgA immune complex in the mesangial area and by histological evidence of mesangial proliferation. In the most severe cases, IgAN may progress to end-stage renal disease in an average time of 10.2 months.Citation1 The loss of glomerular visceral epithelial cells (podocytes) is reportedly associated with the development of glomerulosclerosis and the loss of renal function. Indeed, podocytes are the target site of glomerular involvement.Citation1 Injured podocytes detach from the glomerular basement membrane (GBM) and subsequently appear in the urine. Injury to podocytes can lead to mesangial proliferation and mesangial matrix expansion, which are closely related to IgAN progression.Citation2,3
Podocytes present a variety of specific marker proteins. Of these, podocalyxin (PCX) is well established for podocyte characterization. PCX, a CD34-related sialoglycoprotein expressed in the podocyte cell body, serves to keep the filtration slits open by interacting with abundant actin filaments.Citation4 PCX links to the actin cytoskeleton and regulates the structure of podocytes. As podocytes express PCX exclusively, the podocytes can be distinguished by immunohistochemistry with anti-PCX antibodies.Citation5,6 Recent studies revealed that PCX decreases or diminishes in minimal change passive Heymann nephropathy, accompanied with foot process fusion and proteinuria.Citation7 At present, the clinical use of urinary podocyte counts mainly focuses on monitoring diabetic nephropathy and in the differential diagnosis of idiopathic focal segmental glomerulosclerosis (FSGS) and minimal change nephritic syndrome.Citation8,9 Currently, the association of urinary excretion of podocytes with IgAN is unclear. In this study, we explored the association between the urine excretion of podocytes and the severity of IgAN.
SUBJECTS AND METHODS
From September 2008 to February 2009, 50 patients (18 males, 32 females; age range 14–64 years; mean age 31.9 ± 12.5 years) diagnosed with IgAN by renal biopsy in accordance with the diagnostic criteria for glomerulonephritisCitation10 were recruited for the study. Secondary IgAN cases were excluded. Healthy hospital staff members were used as healthy controls. The study was approved by the Ethical Committee of The Second Xiangya Hospital of Central South University. Informed consent was obtained from all patients.
Urine Sample Collection and Smear Preparation
Morning urine samples were collected for 3 consecutive days before renal biopsy (100 mL/day). Freshly voided urine (100 mL) samples were centrifuged at 700 × g for 5 min. The supernatant was carefully aspirated and the sediment pellet was washed twice with human diploid fibroblast (HDF) solution (distilled water, 137 mM NaCl, 5 mM KCl, 5.5 mM glucose, 4 mM NaHCO3, and 0.2% EDTA). The pellet was then resuspended in 1 mL of HDF. One hundred microliters of resuspended sediment was then centrifuged onto polylysine-coated slides using a TXD3 cell smear centrifuge (Centrifuge Instrument Inc., Xiangyi, China). The slides were air-dried and then fixed with 3% paraformaldehyde at room temperature for 15 min, followed by cell permeabilization using TritonX-100 (Sigma-Aldrich Corp., St. Louis, MO, USA). After washing with phosphate-buffered saline (PBS), the slides were incubated with blocking buffer (PBS containing 0.2% BSA, 50 mM NH4Cl, and 1% goat serum) overnight at 4°C. For each patient, two smears were made each day, and a total of six smears were made and the average number of urinary podocytes in the six smears was calculated. The number of podocytes in each sample was expressed as a ratio to the urinary creatinine content of that sample.Citation11
Immunocytochemical Staining
Slides were incubated with 3% H2O2 for 20 min, blocked with 10% skim milk for 20 min at room temperature, incubated with 1:10 diluted mouse anti-human PCX antibody (R&D Systems, Minneapolis, MN, USA) at 4°C overnight, and then incubated with biotinylated secondary antibody (Maixin Inc., Fuzhou, China) for 30 min at room temperature. After incubation with streptavidin biotin-peroxidase solution for 30 min at room temperature, the slides were subject to 3,3-diaminobenzidine (DAB) staining and hematoxylin counterstaining, and then mounted with glycerol buffer. Except for the 10% skim milk blocking step, all steps were subject to 5 min × 3 PBS wash.
Results and Observations
Brownish staining in cytoplasm was considered positive. All nuclei were stained blue. Podocytes were identified and photographed under high power microscope. Cell counting was performed randomly in each slide in a 200× power under an optical microscope.
PCX Immunohistochemical Staining and Electron Microscopy Examination of Renal Tissue
Renal tissue samples from 50 IgAN patients were formalin-fixed, paraffin-embedded, and immunostained for PCX using an SP immunohistochemical kit (Zhongshan Biological Technology Inc., Beijing, China) according to the manufacturer’s instructions. Freshly prepared DAB solution was used in all experiments. Cell nuclei were counterstained with hematoxylin. A negative control was set up by replacing the primary antibody with PBS in each experiment. In each section, glomeruli in 10 random high power fields (×200) were observed and recorded. Photographs were analyzed by Image-Pro Plus version 6.0 image analysis system (Media Cybernetics, Inc., Bethesda, MD, USA). The hue intensive saturation mode was used to differentiate the positive-stained cells from the background. The average optical density of podocyte PCX staining was calculated by dividing the integrated optical density with the measurement area (A = IOD/area).
Renal tissue samples from 50 IgAN patients were fixed in 2.5% glutaraldehyde and 1% osmium tetroxide and embedded in acid epoxy resin. The morphology of podocytes was observed in ultrathin slices by transmission electron microscopy (TEM) (HITACHI-7500, Hitachi, Japan).
Lee’s Grade and Katafuchi Semiquantitative Integration of Renal Nephropathy
All of the 50 renal biopsy samples were evaluated using both optical microscopy (HE, PAS, PASM, Masson staining) and immunofluorescence (IgG, IgA, IgM, C3, C4, Clq, FRA, HBsAg). Lee SMK’s IgAN histological grade was used as a single integration scheme; Katafuchi semiquantitative integration method was used as a multi-integration scheme. Pathological scoring was performed in a double-blind manner. As the sample sizes in Lee’s pathological classification groups I and II were small, and there was no statistical significant difference between them, the two groups were consolidated into one group in this study.
Statistical Methods
Statistical analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). Measurement data were expressed as mean ± SD or median. For comparison among multiple groups, χ2-test was used for categorical variables, and Kruskal–Wallis test or one-way analysis of variance (ANOVA) was used for continuous variables. Least significance difference method or Mann–Whitney U test was used for pairwise comparisons among multiple groups. Simple correlation was performed with Spearman’s correlation analysis. Continuous variables with non-normal distribution, such as number of urinary podocytes and renal PCX expression, were analyzed after log transformation. The significance level of this study was set at two-sided α = 0.05.
RESULTS
As shown in and , there was no statistically significant difference in age and sex among the Lee’s grade groups of IgAN patients (p > 0.05). However, as shown in , age was positively correlated with Lee’s grade of IgAN.
Table 1. Comparisons of age and sex constitution in Lee’s gradation groups in IgAN patients.
Table 2. Comparisons of clinical dates in Lee’s gradation groups in IgAN patients.
Comparison of Urinary Podocyte Count
No urine podocyte was found in the healthy controls. The urinary podocyte count of IgAN group was significantly higher than that in the healthy control group. There was significant difference in urinary podocyte count among the Lee’s grade groups. Multiple comparison among groups showed that the median of urinary podocytes in Lee’s I–II group was lower than that in Lee’s III, IV, and V groups (p < 0.05); group III lower than group V (p < 0.05). There was no statistically significant difference between Lee’s grade group V and group III or IV. The positive rate of urinary podocytes was highest in Lee’s IV and V groups (100%), and lowest in Lee’s I–II groups (; ).
Figure 1. The comparison of the number of urinary podocytes (10 × 20). (A) Control group. (B) IgAN patient group. [(B1–B5) represent the comparison of the number of podocytes in Lee’s I–V groups in IgAN]. Arrows indicate the representative podocytes.
![Figure 1. The comparison of the number of urinary podocytes (10 × 20). (A) Control group. (B) IgAN patient group. [(B1–B5) represent the comparison of the number of podocytes in Lee’s I–V groups in IgAN]. Arrows indicate the representative podocytes.](/cms/asset/c0f933f4-1ba3-4e17-b3ae-1bc45e868f49/irnf_a_643352_f0001_b.jpg)
Table 3. Comparison of urinary podocytes excretion count.
Comparison of PCX Expression in Lee’s Grade Groups
There was significant difference in the PCX expression among Lee’s grade groups. Multiple comparison among groups showed that the PCX expression in Lee’s I–II grade group (0.092 ± 0.019) was higher than that in Lee’s III (0.053 ± 0.024), IV (0.045 ± 0.014), and V grade groups (0.028 ± 0.007) (p < 0.05). There was significant difference in the PCX expression between Lee’s III grade group and Lee’s IV and V grade groups (p < 0.05). There were no significant differences between Lee’ III and IV grade groups (p > 0.05) ().
Figure 2. Electron microscopy studies in podocyte and PCX expression in Lee’s classification. (A1–A5) represent the morphology of podocyte and foot process changes in Lee’s I–V grades of IgAN (electron microscopy, ×10,000). Electron microscopy shows varying degrees of matrix and mesangial proliferation and a series of podocyte changes, including podocyte body swelling and vacuoles of varying sizes in cytoplasm [(A1) indicated by red arrow]; irregular shape of foot processes with enlargement in the end of primary processes and adhesion to capillary basement membrane, or large amounts of tiny finger-like protrusions in second processes, namely “the formation of microvillus” [(A2) indicated by red arrow]; foot processes adhesion and emerging into high-density electron mass attached to the outside of the basement membrane [(A3) indicated by red arrow]; and podocyte foot strip or fall off from glomerular basal membrane [(A4, A5) indicated by red arrow]. (B1–B5) show glomerular PCX expression in Lee’s I–V grade groups (optical microscope ×200). In Lee’s I grade group, PCX was stained brown and evenly distributed along the glomerular capillary loops; no PCX expression was detected in renal tubules and Bowman’s capsule. In Lee’s grade groups II–V, glomerular PCX expression was unevenly distributed with significant regional decrease of staining, or segmental loss of staining, or total loss of staining in the crescent formation area or the sclerosis area.
![Figure 2. Electron microscopy studies in podocyte and PCX expression in Lee’s classification. (A1–A5) represent the morphology of podocyte and foot process changes in Lee’s I–V grades of IgAN (electron microscopy, ×10,000). Electron microscopy shows varying degrees of matrix and mesangial proliferation and a series of podocyte changes, including podocyte body swelling and vacuoles of varying sizes in cytoplasm [(A1) indicated by red arrow]; irregular shape of foot processes with enlargement in the end of primary processes and adhesion to capillary basement membrane, or large amounts of tiny finger-like protrusions in second processes, namely “the formation of microvillus” [(A2) indicated by red arrow]; foot processes adhesion and emerging into high-density electron mass attached to the outside of the basement membrane [(A3) indicated by red arrow]; and podocyte foot strip or fall off from glomerular basal membrane [(A4, A5) indicated by red arrow]. (B1–B5) show glomerular PCX expression in Lee’s I–V grade groups (optical microscope ×200). In Lee’s I grade group, PCX was stained brown and evenly distributed along the glomerular capillary loops; no PCX expression was detected in renal tubules and Bowman’s capsule. In Lee’s grade groups II–V, glomerular PCX expression was unevenly distributed with significant regional decrease of staining, or segmental loss of staining, or total loss of staining in the crescent formation area or the sclerosis area.](/cms/asset/dcc0e171-863a-4442-8f7c-4c57b430fb52/irnf_a_643352_f0002_b.jpg)
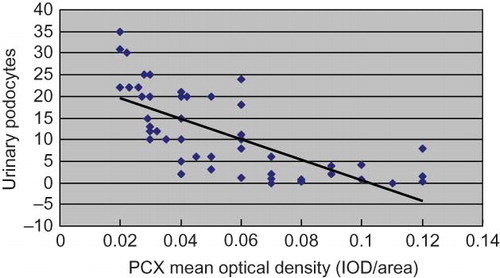
Correlation Analysis between the Urinary Podocyte Counts and the Glomerular Expression of PCX in IgAN Patients
The urinary podocyte count of IgAN patients correlated negatively with the glomerular expression of PCX (r = −0.072, p < 0.01) ().
Correlation Analysis among Urinary Podocyte Count, Expression of PCX, and Clinicopathological Variables of IgAN
As shown in , each glomerular and tubulointerstitial pathological indicator was scored using the Katafuchi semiquantitative integration. The relationship of urinary podocyte count and the expression of PCX with the clinicopathological variables of IgAN is shown in .
Table 4. Comparison of glomerular and tubular pathological scores in Lee’s various renal pathological classification ( ± s).
Table 5. Correlation analysis between the urinary podocyte excretion and the expression of PCX with the clinical dates and pathological scores.
DISCUSSION
Along with the growing understanding of the pathogenesis of IgAN, a number of biomarkers discovered in laboratory studies have been examined in clinical trials. Among these, there are urinary biomarkers useful for screening subjects with suspected IgAN. Podocytes excretion, hematuria, and proteinuria can all be correlated with acute histological injury in children with IgAN, while continued podocyte excretion is correlated with chronic injury and the development of glomerulosclerosis.Citation12 Along these lines, some believe that the urinary podocyte count could predict disease progression in conditions such as lupus nephritis and FSGS.Citation12 In this study, we found significant differences among urinary podocyte counts of the Lee’s grade groups in IgAN patients. That is, the median of urinary podocyte count in Lee’s groups III, IV, and V was higher than that in Lee’s groups I–II. To the extent that this indicates ongoing injury to podocytes in patients with IgAN, it is suggested that the urinary podocyte count could help predict disease progression in those with IgAN. In our research, the positive rate of urinary podocytes in IgAN patients was 88%, significantly higher than that reported in Li’s research (50%).Citation13 This may be the immunohistochemical staining used in this study was more sensitive than indirect immunofluorescence staining. Hara et al.Citation14 found urinary podocytes detached from the capillary loops in active IgAN and suggested that urinary podocyte counts could be an index of active IgAN. Vogelmann et al.Citation11 suggested that the urinary podocyte count was correlated with the disease activity and phenotype of IgAN. In this study, we found that the number of urinary podocytes was increased in Lee’s IV and V groups of IgAN patients and positively correlated with 24-h urinary protein. We demonstrated that the urinary podocyte counts could be used to predict the disease phenotype and activity of IgAN.
Phenotypic changes occur when the podocytes are injured. These include retraction, fusion, and even disappearance of foot processes.Citation15 Podocytes are highly differentiated terminal cells which cannot be regenerated. When injured podocytes shed from the GBM, the exposed area of GBM can only be filled by compensatory hypertrophy of the surrounding podocytes. When the decompensation of hypertrophic podocytes occurs under the capillary hydrostatic pressure, the exposed areas of GBM convex to the renal capsule may then adhere to glomerular parietal epithelial cells, resulting in glomerulosclerosis.Citation15,16 We performed immunohistochemistry staining of renal tissues from patients with different grades of IgAN, and analyzed the positive expression of PCX to evaluate the injury degree of podocytes. Our results showed that PCX antibody specifically bound to podocytes, indicating that the PCX positive cells excreted in urine were glomerular podocytes. Moreover, the positive PCX expression in IgAN patients’ renal tissue decreased with increasing Lee’s grades. The immunohistochemistry staining and TEM results () also suggest that the podocyte injury of IgAN patients is closely associated with disease progression. Previous studies showed that the quantity and duration of proteinuria were associated with disease progression of IgAN.Citation17 The possible mechanisms of the proteinuria include injury to glomerular podocytes with subsequent injury to mesangial cells and tubular epithelial cells eventually leading to glomerulosclerosis and renal interstitial fibrosis.Citation18,19 In this study, we found a negative correlation between glomerular PCX expression and 24-h urinary protein or glomerular and renal tubular pathological scores in IgAN patients. The results showed that decreased glomerular PCX expression was associated with the occurrence of proteinuria, mesangial proliferation, glomerulosclerosis, and tubular atrophy. In line with the report by Yu et al.Citation20 that urinary podocyte counts could directly reflect the severity of renal podocytes injury, our results showed a negative correlation between urinary podocyte counts and the positive PCX expression in glomerular podocytes of IgAN patients.
In summary, our findings suggest that the urinary podocyte count may be an effective, noninvasive test to show the progression of IgAN with high specificity and convenience. Podocyte injury may be an important factor for pathogenesis and progression of IgAN.
ACKNOWLEDGMENT
Funding. This study was supported by the National Natural Science Foundation of China (81170663) to Dr. You-ming Peng.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259.
- Lemely KV, Lafayette RA, Ssfai M, . Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1478.
- Macconi D, Bonomelli M, Benigni A, . Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol. 2006;168:42–54.
- Orlando RA, Takeda T, Zak B, . The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol. 2001;12:1589–1598.
- Doyonnas R, Kershaw DB, Duhme C, . Anuria, omphalocele and perinatal lethality in mice lacking the CD34 related protein podocalyxin. J Exp Med. 2001;194:13–27.
- Takeda T. Podocyte cytoskeleton is connected to the integral membrane protein podocalyxin through Na+/H+-exchanger regulatory factor 2 and ezrin. Clin Exp Nephrol. 2003;7:260–269.
- Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015.
- Nakamura T, Ushiyama C, Suzuki S, . Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant. 2000;15:1379–1383.
- Nakamura T, Ushiyama C, Suzuki S, . The urinary podocyte as a marker for the differential diagnosis of idiopathic focal glomerulosclerosis and minimal-change nephrotic syndrome. Am J Nephrol. 2000;20:175–179.
- Zhou WZ. Diagnostic criteria guidance for renal biopsy. Chin J Nephrol. 2001;8:270–275.
- Vogelmann SU, Nelson JW, Myers BD, . Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–F48.
- Hara M, Yanagihara T, Takada T, . Urinary excretion of podocytes reflects disease activity in children with glomerulonephritis. Am J Nephrol. 1998;18:35–41.
- Li JZ, Huang HC, Liu Y. Significance of detecting urinary podocytes in patients with active glomerulonephritis. J Peking Univ (Health Sci). 2005;37:463–466.
- Hara M, Yanagihara T, Takada T, . Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron. 1995;69:397–403.
- Holzman LB, St John PL, Kovari IA, . Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56:1481–1491.
- Camici M. Urinary detection of podocyte injury. Biomed Pharmacother. 2007;61:245–249.
- Eiro M, Katoh T, Kuriki M, . The product of duration and proteinuria (proteinuria index) is a possible marker for glomerular and tubulointerstitial damage in IgA nephropathy. Nephron. 2002;90:432–441.
- Koop K, Eikmans M, Baelde HJ, . Expression of podocyte associated molecules in acquired human kidney diseases. J Am Soc Nephrol. 2003;14:2063–2071.
- Benigni A, Gagliardini E, Remuzzi G. Changes in glomerular perm-selectivity induced by angiotensin II imply podocyte dysfunction and slit diaphragm protein rearrangement. Semin Nephrol. 2004;24:131–140.
- Yu J, Zou MS, Nie GM, . Effect of lotensin on proteinuria and urinary podocytes in diabetic nephropathy rats. J Clin Res. 2006;23:1366–1369.