Abstract
Background/Aims: Nephrotoxicity induced by aminoglycosides (AGs) limits their clinical use. As yet, no molecules have been approved to prevent AG nephropathy. We aim to investigate the effectiveness of grape seed proanthocyanidin extract (GSPE) in the prevention of amikacin (AK)-induced nephrotoxicity. Methods: A total of 24 rats were allocated into control, GSPE, AK, and AK + GSPE groups. While 1 mL saline was administered for 6 days in control and AK groups, 100 mg/kg GSPE was administered in GSPE and AK + GSPE groups. On day 7, intraperitoneal (i.p.) saline was administered in control and GSPE groups, while 1.2 g/kg i.p. AK was administered in AK and AK + GSPE groups. The experiment was terminated on day 9. Blood samples were taken for the measurement of renal functions. Renal tissues of the rats were removed for the analysis of malondialdehyde (MDA), total oxidant system (TOS), total antioxidant system, oxidative stress index (OSI), and for histopathological examination. Results: MDA level was found to be lower in GSPE group compared with other study groups. There was significantly more renal histopathological damage and higher blood urea nitrogen, creatinine, TOS, OSI, and MDA levels in the AK group compared with the control and AK + GSPE groups. The same parameters showed significant improvement in AK + GSPE group compared with AK group. Conclusion: Our findings demonstrate for the first time that GSPE reduces oxidative damage in AK nephropathy and provides biochemical and renal histopathological improvements.
INTRODUCTION
Aminoglycoside (AG) antibiotics are often used in routine clinical practice for the treatment of gram-negative infections.Citation1 Amikacin (AK) and gentamicin are the two most frequently used AG group antibiotics.Citation2 Despite several advantages of AGs, including high antibacterial efficacy, rapid onset of action, and low cost, they are associated with significant dose-dependent nephrotoxic effects. Nephrotoxicity and autotoxicity limit their clinical use and may lead to significant morbidity and mortality.Citation3 Free oxygen radical damage has often been implicated as the biological basis of AG-induced nephrotoxicity.Citation4
Several experimental studies have been conducted in order to find a way to reduce AG-induced toxicity, and the effects of antioxidant molecules on reducing oxidative damage have been investigated in most of these studies.Citation3–11 It has been demonstrated that oxidative damage can be partially reduced by these drugs. However, there are as yet no molecules approved as an effective method to prevent AG nephropathy in clinical practice.
Proanthocyanidin is a polyphenolic component naturally found in vegetables, fruits, tree barks, flowers, and the seeds of certain fruits. Grape seed proanthocyanidin is a biologically active polyphenolic flavonoid combination containing oligomeric proanthocyanidin.Citation12 Grape seed proanthocyanidin extract (GSPE) has been shown to have antioxidant effects in various in vivo and in vitro studies.Citation12,13
For the first time in the literature, we aimed to investigate the effectiveness of GSPE in the prevention of AK-induced nephropathy by monitoring changes in blood urea nitrogen (BUN), creatinine, as well as renal histopathological changes. We also examined renal total oxidant system (TOS), total antioxidant system (TAS), oxidative stress index (OSI), and malondialdehyde (MDA) levels in order to understand whether the damage occurs via the oxidative system.
MATERIALS AND METHODS
A total of 24 female Sprague-Dawley rats weighing 200–250 g were used in this study. The rats were kept in cages at 22 ± 2°C, with a 12 h/12 h light–dark cycle, and were provided with food and water according to their needs. This study was approved by the Karadeniz Technical University School of Medicine Animal Ethics Committee. Experimental animals were cared for and used in accordance with the National Institute of Health Guide.
Chemicals
GSPE (0.5 mL of extract solution containing 100 mg of GSPE and 66.7 mg/g of total phenolic substance, with an oligomeric proanthocyanidin ratio of 95%) was obtained from Kale Natural Herbal Products Food, Cosmetic and Agricultural products Co., Ltd., Edremit, Balikesir, Turkey.
AK (Amikozit® 500 mg vial; Zentiva, Istanbul, Turkey).
Ketamine (Ketalar® 500 mg injectable vial, Pfizer, Istanbul, Turkey).
Xylazine (Rompun® 50 mL vial, Bayer, Istanbul, Turkey).
Study Protocol
A total of 24 rats were randomly allocated into groups of six after a 5-day adjustment period. All rats were weighed at baseline and were kept in cages with six rats in each cage. The rats had unlimited access to standard rat food and water throughout this study.
Group 1: Control group (n = 6).
Group 2: GSPE group (n = 6).
Group 3: AK group (n = 6).
Group 4: AK + GSPE group (n = 6).
While 1 mL saline (SF) was administered by gavage for 6 days in control and AK groups, 100 mg/kg GSPE (0.5 mL of SF was added to 0.5 mL of grape seed extract containing 100 mg of GSPE in order to obtain a final 1 mL of gavage solution) was administered by gavage for 6 days in GSPE and AK + GSPE groups.
On day 7, intraperitoneal (i.p.) SF was administered in control and GSPE groups, while 1.2 g/kg i.p. AK was administered in AK and AK + GSPE groups.Citation3 After gavage feeding of all rats for 2 more days, the experiment was terminated on day 9 (48 h after the administration of AK). Following induction of anesthesia by administration of 90 mg/kg ketamine and 10 mg/kg xylazine, a midline incision was made. Both kidneys were removed quickly and a sample of 2 mL of blood was collected by cardiac puncture for biochemical analysis. Subsequently, the experiment was terminated by exsanguination.
Biochemical Analysis
BUN and creatinine measurements were made using ROCHE autoanalyzer (Modular System, GmbH, Mannheim, Germany). Following decapsulation of kidneys, longitudinal dissection was performed. Half of the tissue samples were stored at −80°C until the MDA, TAS, TOS, and the OSI levels were measured. At the time of analysis, rat kidney tissues were weighed and homogenized in an ice-cold 1.15% KCl solution containing 0.50 mL/Triton-X-100 by using ULTRA TURRAX T18 basic homogenizer (IKA, Wilmington, NC, USA). The homogenate was centrifuged at 3220 × g for 5 min at 4°C and supernatant was used for the determination of MDA, TAS, and TOS levels.
Tissue MDA levels were assigned according to the method of Uchiyama and Mihara.Citation14 Tetramethoxypropane was used as a standard and tissue MDA levels were given as nmol/g of wet tissue.
Tissue TAS, TOS, and OSI levels were assayed as described previouslyCitation15 by using commercial assay kits (Rel Assay Diagnostics, Gaziantep, Turkey, product numbers: RL0017 and RL0024). Tissue TAS levels were given as μmol Trolox equiv./g of wet tissue and TOS levels were given as μmol H2O2 equiv./g of wet tissue. The OSI values were calculated by using the following formula: [(TOS, μmol H2O2 equiv./g of wet tissue)/(TAS, μmol Trolox equiv./g of wet tissue)] × 100.
Histopathological Evaluation
The other half of the decapsulated kidneys were used for pathological examination. The specimens were fixed by using 10% formalin solution. For light microscopy, they were dehydrated using 70% and 100% alcohol solutions, processed in an autotechnicon, and embedded in paraffin. Sections of 4 μm thickness were cut with a microtome and stained by hematoxylin–eosin, periodic acid Schiff, and Masson’s trichrome. Stained specimens were examined by blinded pathologist using an Olympus BX50 (Olympus, Tokyo, Japan) light microscope. The following scoring system, introduced by Houghton et al., Citation16 was used for the histopathological evaluation of tissues under light microscopy:
Grade 0: Normal.
Grade 1: Desquamation in tubular epithelial cells in small foci (less than 1% of the total tubule population).
Grade 2: Significant tubular cell desquamation or necrosis (less than 50% of cortical tubules).
Grade 3: More than 50% of the proximal tubules showing necrosis, but intact tubules are easily identified.
Grade 4: Complete or almost complete proximal tubular necrosis.
Statistical Analysis
Statistical analysis of data was performed using Statistical Package for the Social Sciences (SPSS) version 13 (SPSS, Chicago, IL, USA), with results expressed as mean ± SD. Groups were examined for normal distribution. Then, Kruskal–Wallis variance analysis and the Mann–Whitney U-test were used for the comparison of BUN, creatinine, MDA, TOS, TAS, OSI, and renal pathological findings. p-Values of less than 0.05 were considered to be statistically significant.
Table 1. The mean values of biochemical and oxidative parameters.
RESULTS
The Effects of Grape Seed Extract on Renal Functions
As presented in , BUN and creatinine levels were significantly higher in the AK group compared with the control group (p = 0.004 and p = 0.02, respectively). BUN and creatinine levels were lower in the AK + GSPE group compared with the AK group. While the BUN level was statistically significantly lower compared with the AK group (p = 0.020), the difference in creatinine level between these groups was not statistically significant (p = 0.055) (). No statistically significant difference was noted between the AK + GSPE group and the control group in terms of BUN and creatinine levels (p = 0.724 and p = 0.229, respectively) ().
The Effect of Grape Seed Extract on Oxidative Damage
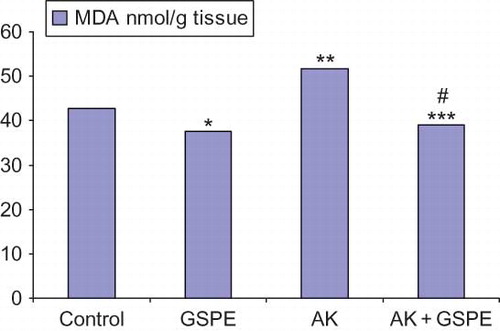
As seen in , MDA, TOS, and OSI levels were highest in the AK group. The MDA level in the GSPE group was significantly lower compared with the control group (p = 0.025). The MDA, TOS, and OSI levels in the AK group were significantly higher compared with the control group (p = 0.010, p = 0.024, and p = 0.004, respectively) (). The MDA, TOS, and OSI levels in the AK + GSPE group were significantly lower compared with the AK group (p = 0.004, p = 0.024, and p = 0.004, respectively) (; ).
The Effect of Grape Seed Extract on Renal Histopathology
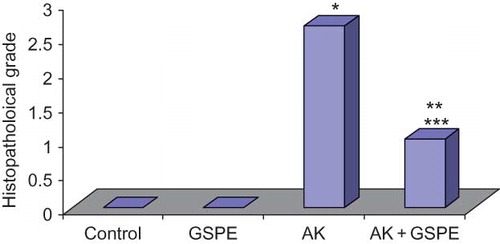
Whereas there was Grade 3 damage in five rats and Grade 1 damage in one rat in the AK group, there was Grade 0 damage in two rats, Grade 1 damage in two rats, and Grade 2 damage in two rats in the AK + GSPE group (; A–C). This decreased renal histopathological damage in the GSPE supplementation group was statistically significant.
Table 2. Distribution of renal pathological scores in the study groups.
When renal histopathological changes were compared between the study groups, significant pathological change was noted in the AK group compared with the control group (p = 0.001). Significantly less damage was seen in the AK + GSPE group compared with the AK group (p = 0.012) ().
DISCUSSION
AG antibiotics are frequently used in daily practice for the treatment of gram-negative infections due to several advantages, including chemical stability, rapid bactericidal effect, low cost, synergistic effect with beta-lactam antibiotics, and lower potential for the development of resistance.Citation17 However, the nephrotoxicity associated with their clinical use causes significant morbidity and mortality.Citation3 As these antibiotics are not metabolized in the body and have low affinity to proteins, most of the drugs found in circulation are filtrated through the glomeruli without going through any change, and about 10% are reabsorbed from the tubuli and accumulate in the renal cortex. Tubular deposition of these drugs results in high plasma concentrations and prolongation of their half lives.Citation1,4
AK-induced proximal tubular damage begins with tubular uptake. The cationic structure of the molecule facilitates its intracellular uptake.Citation18 Polyanionic inositol membrane phospholipids are major binding regions for AG and megalin, a member of the lipoprotein family located in the proximal cell membrane that has been recently shown to play a role in intracellular uptake, especially by endocytosis.Citation1 Several studies have investigated whether nephropathy can be prevented by reducing tubular uptake of AG. In one of these studies, Antoine et al.Citation19 have experimentally shown that statins reduce tubular uptake of AG. In another study, Bennett et al.Citation20 demonstrated that increased dietary calcium load reduces tubular uptake of gentamicin.
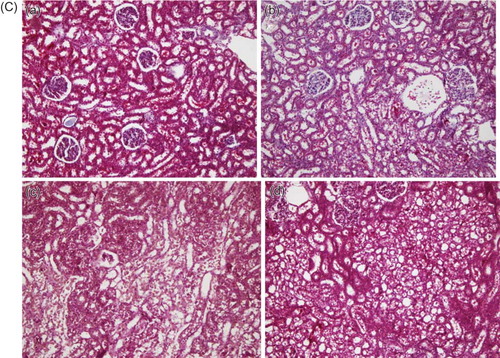
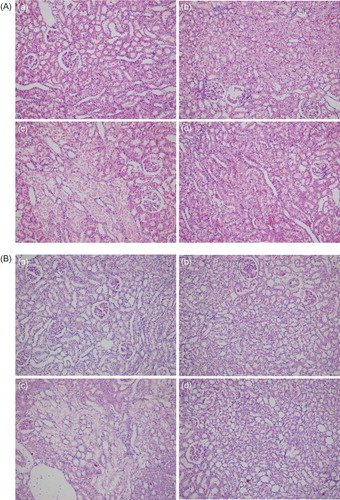
Figure 2. (A) Histopathological image of groups, using hematoxylin–eosin (HE). (a) Control, (b) GSPE alone, (c) AK, and (d) GSPE + AK. No pathological findings were noted in renal samples from GSPE and control groups. Kidney with normal appearance (HE ×200). AK: Extensive necrosis and desquamation noted in renal proximal tubular cells. Glomeruli are intact (HE ×200). AK + GSPE: AK + GSPE-treated rats showing prevention of AK-induced alterations. Only mild granulovacuolar changes are noted in renal proximal tubular epithelial cells (HE ×200). (B) Histopathological image of groups, using periodic acid Schiff (PAS). (a) Control, (b) GSPE alone, (c) AK, and (d) GSPE + AK. No pathological findings were noted in renal samples from GSPE and control groups. Kidney with normal appearance (PAS ×200). AK: Extensive necrosis and desquamation noted in renal proximal tubular cells. Glomeruli are intact (PAS ×200). AK + GSPE: AK + GSPE-treated rats showing prevention of AK-induced alterations (PAS ×200). (C) Histopathological image of groups, using Masson’s trichrome. (a) Control, (b) GSPE alone, (c) AK and (d) GSPE + AK. No pathological findings were noted in renal samples from GSPE and control groups. Kidney with normal appearance (Masson’s trichrome ×200). AK: Extensive necrosis and desquamation noted in renal proximal tubular cells. Glomeruli are intact (Masson’s trichrome ×200). AK + GSPE: AK + GSPE treated rats showing prevention of AK-induced alterations (Masson’s trichrome ×200).
Note: AK, amikacin; GSPE, grape seed proanthocyanidin extract.
After intracellular uptake, AG accumulates in lysosomes, the golgi apparatus, and the endoplasmic reticulum. Following lysosomal deposition, the lysosomes expand due to excessive lipid debris, resulting in the appearance of classical myelin figures on electron microscopy. In a previous study, we demonstrated myelin figure formation induced by AK administration in mice.Citation9 This leads to fragmentation of these organelles and cytoplasmic release of their contents, which contributes to apoptosis and necrosis in the cell. Furthermore, AG released into the cytoplasm increases the formation of superoxide anions and hydroxyl radicals within the mitochondria, leading to the formation of reactive oxygen species (ROS) and associated damage.Citation21 This in turn causes an increase in MDA level, which is an indicator of ROS lipid peroxidation.
Although tubular damage is the major pathology underlying AG nephropathy, glomerular and vascular involvement is also present. Increased glomerular permeability leads to enhanced mesangial cell contraction and decreased renal blood flow. However, in light of current evidence, the most widely accepted mechanism underlying the pathogenesis of AG nephrotoxicity is oxidative damage and its associated tubular necrosis.Citation1,17 In this study, we observed significant increase in BUN and creatinine levels, biochemical indicators of renal function, as well as Grade 3 histopathological changes in renal tissue samples following a single i.p. dose of 1.2 g/kg AK injection. Moreover, we have also noted that MDA, TOS, and OSI levels, indicators of oxidative damage, were significantly higher in the AK group compared with the controls. Our findings support the hypothesis that AK-induced nephropathy occurs via an oxidative mechanism.
Experimental studies have been performed using several antioxidant molecules in an attempt to reduce the damage observed in AG-induced nephropathy. Gentamicin nephropathy models have been more extensively studied, using molecules including fish oil,Citation4 pentoxifylline,Citation5 green tea,Citation6 caffeic acid phenyl ester (CAPE),Citation7 vitamin E and vitamin C,Citation8 and GSPE,Citation11 and so on. The AK nephropathy model has been used in fewer studies, but the effects of antioxidant molecules including CAPE,Citation3 N-acetylcysteine (NAC),Citation9 melatonin,Citation22 and pentoxifylline Citation10 have been investigated. While alterations in oxidative system, as well as biochemical and histopathological changes have been examined in some of the studies, only biochemical and histopathological changes have been investigated in others. It has been shown experimentally in these studies that antioxidant molecules can lead to some degree of improvement in nephropathy. However, they have not been introduced into clinical practice because they have not yet been proven to prevent AG nephropathy. In this study, we aimed to investigate the effects of GSPE on an AK nephropathy model for the first time.
Figure 3. Comparison of histopathological grades between the study groups. Notes: *AK versus control, p = 0.001. **AK + GSPE versus control, p = 0.022. ***AK versus AK + GSPE, p = 0012.
GSPE is a biologically active polyphenolic flavonoid combination containing oligomeric proanthocyanidin.Citation13 Proanthocyanidins are strong antioxidant molecules, and are polyphenolic compounds naturally present in many vegetables and fruits.Citation12 In addition to ROS scavenging and antioxidant effects, it has been demonstrated in various experimental studies that GSPE has vasodilator, anti-carcinogenic, anti-allergic, anti-inflammatory, antibacterial, cardioprotective, immunomodulatory, and antiviral effects.Citation12,13 In an in vitro study investigating its antioxidant effects, 100 mg/kg GSPE has been found to have a much stronger antioxidant effect than vitamin E and vitamin C.Citation23 Its organ-specific protection effects have been demonstrated in acetaminophen-induced hepatotoxicity, amiodarone-induced pulmonary toxicity, doxorubicin-induced cardiotoxicity,Citation24 and cisplatin-induced renal toxicity.Citation25 Saad et al. have evaluated the protective effect of GSPE in cisplatin-induced nephropathy. They have showed that GSPE use was associated with a reduction in cisplatin nephrotoxicity. The oxidative system was also examined in that study, and while no significant changes were seen in the oxidative system and renal MDA, a lipid peroxidation product was observed in the GSPE-alone group, antioxidant enzyme levels were nevertheless found to be decreased, and MDA level was found to be increased in the cisplatin group. Improvement in antioxidant system and MDA levels was observed in the cisplatin group following the administration of GSPE.Citation25 Yanarateş et al. investigated the protective effect of GSPE on renal ischemic reperfusion injury and similarly showed that GSPE has a protective and oxidative stress-reducing effect. However, because this study lacked a group receiving GSPE alone, the isolated effects of GSPE on the oxidative system could not be evaluated.Citation26 The effects of GSPE on the prevention of AK nephropathy have not been previously investigated. Safa et al.Citation11 investigated its preventive effect on gentamicin nephrotoxicity and found that it provides biochemical and histopathological improvements, but they did not evaluate its effects on the oxidative system. We have examined whether GSPE reduced renal damage by administrating 100 mg/kg GSPE for a total period of 9 days including 7 days prior to 2 days after i.p. AK administration. We then compared the study groups in terms of changes in serum BUN and creatinine levels, histopathological findings, renal MDA, TOS, TAS, and OSI levels. While there were no changes in BUN or creatinine levels, nor were there any renal histopathological findings, in the group receiving GSPE alone, MDA level was significantly lower compared with the control and the other study groups. This finding was different from the results reported by Saad et al.Citation25 We have noted for the first time that GSPE reduced oxidative damage markers in renal tissue of healthy rats. Renal histopathological alteration was noted and there was a significant associated increase in BUN and creatinine levels in the AK group. Furthermore, highest MDA, TOS, and OSI levels indicating oxidative damage were also found in this group. Finally, we noted a statistically significant degree of histopathological improvement in the AK + GSPE group. Also in the AK + GSPE group, BUN, creatinine, MDA, TOS, and OSI levels were improved to such a degree that no significant difference was found compared with the control group.
In conclusion, our findings demonstrate for the first time that GSPE reduces oxidative damage due to AK nephropathy and provides biochemical and histopathological improvements. However, in addition to ROS-induced damage, apoptosis, inflammation, and reduction in renal blood flow are also implicated in the pathogenesis underlying AG nephrotoxicity. It has been suggested in experimental studies that GSPE also has vasodilator, antiapoptotic, and anti-inflammatory features in addition to its antioxidant effect. These additional effects might also have contributed to the biochemical and histopathological improvements noted in renal functions in our study. Future experimental and clinical studies may provide further insight toward the explanation of the beneficial effects of GSPE on AK nephropathy.
Practical Application
We aim to investigate the effectiveness of GSPE in the prevention of AK nephrotoxicity. Our findings support that the damage occurs by an oxidative mechanism. GSPE reduces oxidative damage and provides renal function and histopathological improvement in AK nephropathy in rats. If the findings are supported by human studies, GSPE commonly found in nature may be used in preventing AK nephropathy.
ACKNOWLEDGMENTS
The authors thank Kale Natural Herbal Products Food Cosmetics and Agriculture Ltd., which has provided the grape seed extract used in this study. This study was supported by the Scientific Research Fund of Karadeniz Technical University.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Nagai J, Takano M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet. 2004;19:159–170.
- Sweileh WM. A prospective comparative study of gentamicin- and amikacin-induced nephrotoxicity in patients with normal baseline renal function. Fundam Clin Pharmacol. 2009;23:515–520.
- Parlakpinar H, Ozer MK, Ucar M, . Protective effects of caffeic acid phenethyl ester (CAPE) on amikacin-induced nephrotoxicity in rats. Cell Biochem Funct. 2006;24:363–367.
- Priyamvada S, Priyadarshini M, Arivarasu NA, . Studies on the protective effect of dietary fish oil on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Prostaglandins Leukot Essent Fatty Acids. 2008;78:369–381.
- Stojiljkovic N, Veljkovic S, Mihailovic D, . Protective effects of pentoxifylline treatment on gentamicin-induced nephrotoxicity in rats. Ren Fail. 2009;31:54–61.
- Khan SA, Priyamvada S, Farooq N, Khan S, Khan MW, Yusufi AN. Protective effect of green tea extract on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Pharmacol Res. 2009;59:254–262.
- Parlakpinar H, Tasdemir S, Polat A, . Protective role of caffeic acid phenethyl ester (cape) on gentamicin-induced acute renal toxicity in rats. Toxicology. 2004;207:169–177.
- Kadkhodaee M, Khastar H, Arab HA, Ghaznavi R, Zahmatkesh M, Mahdavi-Mazdeh M. Antioxidant vitamins preserve superoxide dismutase activities in gentamicin-induced nephrotoxicity. Transplant Proc. 2007;39:864–865.
- Kaynar K, Gul S, Ersoz S, Ozdemir F, Ulusoy H, Ulusoy S. Amikacin-induced nephropathy: Is there any protective way? Ren Fail. 2007;29:23–27.
- Ozer MK, Asci H, Oncu M, . Effects of pentoxifylline on amikacin-induced nephrotoxicity in rats. Ren Fail. 2009;31:134–139.
- Safa J, Argani H, Bastani B, . Protective effect of grape seed extract on gentamicin-induced acute kidney injury. Iran J Kidney Dis. 2010;4:285–291.
- Bagchi D, Bagchi M, Stohs S, Ray SD, Sen CK, Preuss HG. Cellular protection with proanthocyanidins derived from grape seeds. Ann N Y Acad Sci. 2002;957:260–270.
- Bagchi D, Sen CK, Ray SD, . Molecular mechanisms of cardioprotection by a novel grape seed proanthocyanidin extract. Mutat Res. 2003;523–524:87–97.
- Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278.
- Harma M, Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly. 2003;133:563–566.
- Houghton DC, Plamp CE 3rd, DeFehr JM, Bennett WM, Porter G, Gilbert D. Gentamicin and tobramycin nephrotoxicity. Am J Pathol. 1978;93:137–152.
- Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011;79:33–45.
- Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: Importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99.
- Antoine DJ, Srivastava A, Pirmohamed M, Park BK. Statins inhibit aminoglycoside accumulation and cytotoxicity to renal proximal tubule cells. Biochem Pharmacol. 2010;79:647–654.
- Bennett WM, Elliott WC, Houghton DC, Gilbert DN, DeFehr J, McCarron DA. Reduction of experimental gentamicin nephrotoxicity in rats by dietary calcium loading. Antimicrob Agents Chemother. 1982;22:508–512.
- Silverblatt F. Pathogenesis of nephrotoxicity of cephalosporins and aminoglycosides: A review of current concepts. Rev Infect Dis. 1982;4:360–365.
- Parlakpinar H, Ozer MK, Sahna E, Vardi N, Cigremis Y, Acet A. Amikacin-induced acute renal injury in rats: Protective role of melatonin. J Pineal Res. 2003;35:85–90.
- Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179–189.
- Ray SD, Patel D, Wong V, Bagchi D. In vivo protection of DNA damage associated apoptotic and necrotic cell deaths during acetaminophen-induced nephrotoxicity, amiodarone-induced lung toxicity and doxorubicin-induced cardiotoxicity by a novel IH636 grape seed proanthocyanidin extract. Res Commun Mol Pathol Pharmacol. 2000;107:137–166.
- Saad AA, Youssef MI, El-Shennawy LK. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: The protective effect of grape seed proanthocyanidin extract. Food Chem Toxicol. 2009;47:1499–1506.
- Yanarates O, Guven A, Sizlan A, . Ameliorative effects of proanthocyanidin on renal ischemia/reperfusion injury. Ren Fail. 2008; 30: 931–938.