Abstract
Background: To determine whether the type of renal artery stenosis and the rapid decline of renal function may have an impact on renal outcome after stenting. Methods: Thirty patients with chronic kidney disease stages 3–4 and renal artery stenosis underwent stenting. The mean follow-up was 33 months; the change of estimated glomerular filtration rate was expressed as negative or positive value in mL/mo (ΔGFR). We identified two types of subgroups, on the basis of stenosis type: 1 (unilateral) N = 13 and 2 (7 bilateral, 2 single kidney, 8 prevalent kidney) N = 17; on the basis of declining ΔGFR in a pre-stenting period of 10 months: slow progressor (N = 11) and fast progressor (N = 13). Results: Thirty-seven stents were placed successfully. After stenting the median ΔGFR value was significantly greater in subgroup 2 compared with subgroup 1 (0.02 vs. −0.16; p = 0.02). Being in fast progressor and in subgroup 2 were associated with improved renal function after stenting (8 of 13 patients, p = 0.013; 11 of 17 patients, p = 0.032). In a logistic regression the only significant relationship is between improvement of renal function and rapid decline of pre-stenting GFR (odds ratio 16; p = 0.005). Conclusion: The predictable benefit from renal stenting may be most likely in patients presenting with a rapid decline of GFR associated with renal artery stenosis affecting the whole renal mass that is both kidneys or single functioning kidney.
INTRODUCTION
Atherosclerotic renovascular disease is found not infrequently in patients older than 45 years old with end-stage renal disease (ESRD). In certain patient populations such as those with severe atherosclerosis in peripheral vascular beds, severe and refractory hypertension, aortic aneurysm, or coronary artery disease the prevalence is as high as 15–33.1%.Citation1
High-grade renal artery stenosis is associated with variable rates of progression toward occlusion based on the type of imaging test used. Nevertheless, in studies that used duplex ultrasonography (DUS),Citation2 the progression of the severity of stenosis over a 5-year period approximates 50% of patients whose lumen narrowing is >60% at the time of the initial detection. In the management of patients with atherosclerotic renal artery stenosis (ARAS) the clinical dilemma is the assessment of the functional relevance of the stenosis, in order to select those patients who are going to be responders to revascularization.
The most crucial issue is to distinguish coincidental ARAS in patient with unrelated chronic kidney disease (CKD) from true ischemic nephropathy. More often large vessel atherosclerotic disease is superimposed on hypertensive microvascular disease, aging, and diabetes mellitus.
Three recent randomized trials, the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC),Citation3 the STent placement in patients with Atherosclerotic Renal artery stenosis (STAR),Citation4 and the Angioplasty and STent for Renal Artery Lesions (ASTRAL),Citation5 comparing renal artery stenting versus medical therapy alone, do not demonstrate any benefit in the stented groups. Currently, the decision about renal revascularization versus medical therapy is clinical, based on estimating likelihood of benefit of the revascularization in each individual patient. The aim of our study is to identify reasonable criteria to select patients with ARAS who could benefit from renal stenting.
MATERIALS AND METHODS
Clinical Setting
The study was performed at one site having 13 practicing nephrologists. All patients referred to our Nephrology Clinic with unexplained renal impairment (n = 616) underwent renal DUS. Other patients were also referred by vascular surgeons (n = 205) preoperatively with atherosclerotic processes (aortic aneurysms, carotid, and peripheral arterial disease) and renal dysfunction (serum creatinine ≥1.5 mg/dL). We ultimately screened 821 patients with CKD stages 3–4 using renal DUS, from January 2003 through December 2008.
Study Variables
The severity of ARAS was classified according to the validated criteria estimated at the site of stenosisCitation6 and on intrarenal arteries distal to the stenosis.Citation7 At the site of stenosis these criteria are based on the highest renal artery peak systolic velocity (PSV) and the renal aortic ratio (RAR), defined as the highest PSV divided by the aortic PSV. The indirect evaluation involves Doppler examination of the segmental or interlobar arteries within the kidney and it permits to detect the presence of post-stenotic flow and to measure the renal resistive index (RI). High-grade stenosis of a feeding artery delays the systolic rise in arteries distal to it. The resulting waveform shape is termed tardus parvus.
Whenever possible we defined diagnosis only using DUS relegating to second line investigation, the use of computed tomography or magnetic resonance angiography, because of their high costs and to avoid gadolinium toxicity and contrast nephropathy.
Renal function was monitored by abbreviated Modification of Diet in Renal Disease method [estimated glomerular filtration rate (eGFR)]. The changes in eGFR before and after stenting were normalized at the month of follow-up and they were expressed as negative or positive change (ΔGFR).Citation8 At least three consecutive creatinine measurements and an observation period longer than 6 months before and after revascularization were required for the assessment of renal function changes. A shorter period could have affected our ability to detect a definitive change in renal function. All patients received medical treatment with statins and aspirin. The termination of follow-up took place with patients alive in CKD or until ESRD or death in CKD. The patients with intrastent restenosis were excluded from the final analysis because the aim of our study was to analyze the change in renal function after stenting and restenosis could influence the rate of progression of renal failure.
Renal size was obtained from ultrasound renal length and in those with bilateral disease the mean size of both kidneys was considered for analysis. The definition of a prevalent kidney required the presence of a contralateral kidney smaller than 8 cm length on ultrasound examination and a length difference between the two kidneys greater than 1.5 cm.
We define the following subgroups based on stenosis type: subgroup 1 (unilateral) and subgroup 2 (bilateral, solitary kidney, prevalent kidney). We included the prevalent kidney into subgroup 2 because most of the renal function comes from one kidney similar to a solitary kidney.
Biochemical data with a pre-stenting observation of a mean period of 10 ± 4 months were available only in 24 patients. On the basis of the rate of decline of renal function before stenting, we identified two other subgroups: slow progressor (N = 11) and fast progressor (N = 13) (ΔGFR cut point used was −0.25 mL/mo).
Blood pressure (BP) assessment was documented by mean values of multiple observations recorded at home before and after the procedure. Proteinuria was graded with a semi-quantitative score from 0 (<300 mg/day) to 1 (0.3–1 g/day) or 2 (>1 g/day). The degree of renal artery lesion was assessed by visual angiographic estimation and it was considered critical if >70%.
The decision to treat renal artery stenosis with stenting was driven largely by the goal of slowing the decline of renal function in patients with impaired renal function. Secondary end points were changes in BP control and total mortality.
Statistical Analysis
The data are expressed as mean ± SD where the distribution is Gaussian or as median and interquartile (IQ) ranges for variables with skewed distributions. Comparisons between groups were performed using paired Student’s t-test or Mann–Whitney test according to the variable distribution. Spearman’s rank correlation test was used for assessing the degree of relationship between not normally distributed variables. Fisher’s exact test was used for calculating an exact probability value for the relationship between two dichotomous variables. Logistic regression was used to analyze the relationship between one dichotomous dependent variable and two or more independent variables. Probability values of <0.05 were considered as statistically significant. Data were analyzed using the statistical package MedCalc for Windows, Version 11.3 (MedCalc Software, Mariakerke, Belgium).
RESULTS
shows the study flow diagram with subjects and findings. A new diagnosis of ARAS, using the DUS criteria, was made in 151 of the 821 patients (19%). In the end we analyzed the data of a total of 37 transluminal stents placed in 30 patients. The baseline characteristics of the 30 patients are demonstrated in .
Figure 1. Study flow diagram.
Note: PSV, peak systolic velocity; RAR, renal aortic ratio; ARAS, atherosclerotic renal artery stenosis.
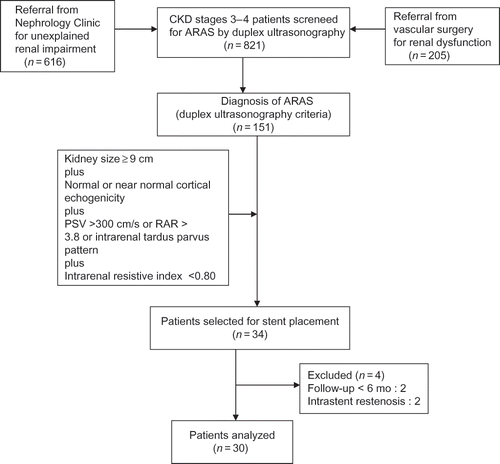
Table 1. Demographic data and comorbid disease at stenting.
Following Percutaneous Transluminal Angioplasty with stenting, artery patency was achieved in all cases. No periprocedural-related death or major complications occurred.
No difference at baseline was noted between the two subgroups of stenosis type (1 vs. 2 and slow progressor vs. fast progressor) for the following variables: age, eGFR, renal length, RI, degree of proteinuria, BP control, diabetes, and prevalence of comorbid vascular diseases.
The mean systolic and diastolic BP did not show any significant change before and after stenting (156 ± 31 mmHg/89 ± 13 mmHg at baseline). A mean of 2.1 ± 1 oral antihypertensive drugs was needed pre-stenting with no change in the number of antihypertensive medications needed after angioplasty.
The follow-up was 33 ± 21 months. Two patients (6.6%) died and seven patients (23.3%) reached ESRD requiring initiation of hemodialysis. The patients who reached ESRD had more advanced renal insufficiency at the time of stenting (CKD stage 4 with a mean eGFR of 20.2 mL/min) and showed a more rapid decline in renal function after stenting (ΔGFR = −1.3 mL/mo).
shows the results of renal outcome in the entire group of patients and in subgroups 1 and 2. The measures of outcome were “improved renal function” (positive change in eGFR), “stabilized renal function” (“physiologic” loss of GFR of about 1.3 mL/year that is equivalent to a ΔGFR between 0 and −0.11 mL/mo), and “worsened renal function” (progression of renal failure).
Table 2. Clinical outcome after stenting at latest follow-up.
We considered improved and stabilized renal function as positive outcomes. Accordingly, 20 patients (66.6%) had a positive result. In a subgroup analysis, we found that a positive renal outcome was achieved in 14 of 17 patients (82%) in subgroup 2, those with bilateral, single, or prevalent kidney ARAS, and only in 6 of 13 patients (46%) in subgroup 1, those with unilateral ARAS. This difference in renal outcome between the two stenosis subgroups is also shown in , where, at latest follow-up, the median value of ΔGFR was positive and significantly greater in subgroup 2 than the negative value in subgroup 1: 0.02 (IQ ranges −0.11 to 0.21) versus −0.16 (IQ ranges −0.83 to 0.03); p = 0.02 (Mann–Whitney test). Belonging to subgroup 2 was also a predictor of long-term improvement of renal function (11 of 17 patients vs. 3 of 13 patients; p = 0.032; Fisher’s exact test).
Figure 2. Difference in estimated glomerular filtration rate [ΔGFR (mL/mo)] after stenting between unilateral (subgroup 1) and bilateral, single, or prevalent kidney atherosclerotic renal artery stenosis (ARAS) (subgroup 2).
![Figure 2. Difference in estimated glomerular filtration rate [ΔGFR (mL/mo)] after stenting between unilateral (subgroup 1) and bilateral, single, or prevalent kidney atherosclerotic renal artery stenosis (ARAS) (subgroup 2).](/cms/asset/5af3a2a1-3236-4d01-9c4f-063ee1a68b27/irnf_a_646807_f0002_b.gif)
Regardless of the type of stenosis, being in the fast progressor subgroup as compared with the slow progressor subgroup was associated with improved renal function after stenting (8 of 13 patients vs. 1 of 11 patients; p = 0.013; Fisher’s exact test). shows the different outcome between fast progressor and slow progressor subgroups: ΔGFR after stenting 0.1 (IQ ranges −0.28 to 0.24) versus −0.14 (IQ ranges −0.89 to 0); p = 0.04 (Mann–Whitney test).
Figure 3. Difference in estimated glomerular filtration rate [ΔGFR (mL/mo)] after stenting between the fast progressor and slow progressor subgroups.
![Figure 3. Difference in estimated glomerular filtration rate [ΔGFR (mL/mo)] after stenting between the fast progressor and slow progressor subgroups.](/cms/asset/5fdac39b-dc27-4371-a526-06e7da984b38/irnf_a_646807_f0003_b.gif)
To determine whether the rate of progression in the basal pre-intervention period influenced the change in GFR after stenting, we examined the correlation between the difference in the pre- and post-stenting slopes (ΔGFR) as a function of the pre-intervention value in the entire cohort of patients. The correlation was negative (r = −0.525; p = 0.008; Spearman’s rank correlation coefficient). The mean value of ΔGFR was significantly greater after than before stent placement only in subgroup 2 (0.05 ± 0.46 vs. −0.63 ± 0.71; p = 0.02; paired t-test) () while the ΔGFR in subgroup 1 did not show any significant change (−0.22 ± 0.26 vs. −0.72 ± 1.34; p = ns; paired t-test).
Figure 4. Changes in estimated glomerular filtration rate [ΔGFR (mL/mo)] before and after stent placement in the bilateral, single, or prevalent kidney atherosclerotic renal artery stenosis (ARAS).
![Figure 4. Changes in estimated glomerular filtration rate [ΔGFR (mL/mo)] before and after stent placement in the bilateral, single, or prevalent kidney atherosclerotic renal artery stenosis (ARAS).](/cms/asset/1447c164-9eba-423c-8175-112b45ac2982/irnf_a_646807_f0004_b.gif)
A negative correlation was also found between the grade of proteinuria and the ΔGFR after stenting (r = −0.520; p = 0.003; Spearman’s rank correlation coefficient). None of the patients with significant proteinuria (6 of 30) achieved improvement of renal function after stenting compared with those with minimal proteinuria, <300 mg/day (p = 0.018; Fisher’s exact test).
Because at least three variables on univariate analysis predicted improvements, we performed a logistic regression analysis. In the model, improvement of renal function (ΔGFR >0) as the dependent variable was examined as a function of the type of stenosis, the rate of decline in the basal pre-stenting period, and the degree of proteinuria as the independent variables. Improvement of renal function correlated with the rate of decline of GFR in the pre-stenting period (odds ratio 16; 95% Confidence Intervals (CI): 1.54–166; p = 0.005), but not with the type of stenosis or grade of proteinuria.
DISCUSSION
The prevalence of ARAS depends on the definition selected, diagnostic tool used, and the population screened. In a high-risk population, as is our cohort of CKD patients with a vascular referral center, the prevalence reported from the literature ranges from 15% to 33.1 %.Citation9 Our data on prevalence (19%), detected using DUS, are in agreement with data reported.
To date a few prospective randomized clinical trials have been completed and all failed to demonstrate benefits of stenting on pertinent renal outcomes compared with aggressive medical therapy alone. In a recent review, Weinberg and OlinCitation10 contend that the three major studies (DRASTIC, STAR, and ASTRAL) are so seriously flawed that it is impossible to make treatment decisions based on their results. The main pitfall is the inclusion in the study of patients whose stenosis was not critical (50% of patients in the STAR trial had mild stenosis and 41% of patients in the ASTRAL trial had stenosis less than 70%). On the other hand, several cohort studies have documented improvements in creatinine and the slope of reciprocal creatinine after stenting in a subset of patients compatible with a beneficial effect on renal function.Citation11,12 A limitation in these longitudinal uncontrolled studies is the relatively small number of patients in each group which prevents a rigorous comparison of possible differences in response to stenting.
According to the data of literature,Citation13–15 we identified in the post-stenting follow-up, three degrees of renal function outcome based on a change in the rate of decline of GFR before and after angioplasty and stenting: “improved”, “worsened”, and “stabilized”. The criteria used to separate the three states were based on epidemiological data and studies of kidney donors which indicate that with age >50 years, the GFR is physiologically reduced to about 1 mL/year (equivalent to 0.08 mL/mo).Citation16 Based on these conclusions and on the data items derived on mean rate of decline of GFR in CKD from different etiologies, we considered stabilized a change of GFR per year of 1.3 mL/year (equivalent to a ΔGFR −0.11 mL/mo).
We tested the hypothesis that the patients with bilateral, single, or prevalent kidney ARAS might have more global severe ischemiaCitation17 than those with simple unilateral ARAS. In our study, we selected high-risk patients coupling an effective hemodynamic stenosis (PSV >300 cm/s or RAR > 3.8 or tardus parvus pattern) in a kidney with a low grade of disease staging (size ≥ 9 cm plus near normal echogenicity plus intrarenal resistive index <0.80). Doppler ultrasound provided additional relevant information on hemodynamic assessment of small vessels.Citation18,19 The measurement of diastolic flow expressed as intrarenal resistive index indicated the staging of parenchymal downstream disease. Radermacher et al.Citation20 showed that a resistive index of >0.80 predicted lack of response in BP control or renal function to revascularization. All our patients had at stenting a favorable resistive index (mean value of 0.66).
Our data support the hypothesis that global ischemia is an important predictor of a good response to revascularization since the benefit from change in GFR was greater in patients with ARAS that affected the whole renal mass. In such patients improved and stabilized renal function as positive outcomes occurred in the great majority of patients (82%). In 14 of 17 patients stented in subgroup 2, a favorable change in the sequential renal function (expressed as ΔGFR) occurred indicating that stent placement may produce a clear improvement in changes of renal function.
Another useful predictive parameter was the grade of proteinuria. Our findings confirm the findings of Wright et al.Citation21 who initially brought attention to the finding that significant proteinuria predicted a poor outcome from revascularization. In our study two patients had proteinuria >1 g/day and both had a rapid progression of renal failure after stent placement. Moreover no patient with significant proteinuria achieved an improvement of renal function after stenting.
The current literature indicates that there is significant heterogeneity in the rate of decline of renal function in patients with ARAS. Better understanding of the natural history of ARAS is essential for rational and effective therapy. The major difficulty is how to separate the fast progressors from the slow progressors since they may benefit differently following revascularization. In a prospective study, Muray et al.Citation22 found that a rise in serum creatinine of more than 0.1 mg/dL/mo before stenting seemed to predict a benefit in the renal function. In a cohort of 51 patients followed for 5 years with bilateral atherosclerotic renovascular disease, Baboolal et al.Citation23 showed that the rate of decline in renal function was variable. The average rate of decline of GFR for the patients who, at latest follow-up, were alive in CKD was 3 mL/min/year (eGFR from 39 to 24 mL/min). Based on these data, we extrapolated that the decline of 3 mL/year (equivalent to 0.25 /mo) could be the expected value for an average loss of renal function in patients with ARAS. So, we considered the cut point of −0.25 mL/mo to separate the slow progressor and fast progressor subgroups.
In the entire group of patients of our study, the rate of declining GFR before stenting showed an inverse correlation with changes of GFR after stenting. In order to test whether recent deterioration in renal function predicts a favorable renal outcome, we then analyzed the outcomes of slow progressor and fast progressor subgroups as defined above. Those patients with a pre-stenting rapid decline, regardless of the type of stenosis, had a better renal outcome after stenting. However when the improvement in GFR following stenting was analyzed as a function of several independent variables by logistic regression, the only independent predictor was the initial rate of decline of GFR in the pre-stenting period indicating that the rapid decline of renal function is the strongest predictor of improvement of renal function after stenting.
We found in our study no change in either BP control or medication requirements. This is in agreement with several randomized studies that have failed to demonstrate any benefit in BP control.Citation24,25
In conclusion, renal stent placement in selected patients may slow the progression of renal impairment of CKD with long-term preservation on renal function. Our data indicate that preservation of renal function may be more likely in patients with ARAS affecting the whole renal mass that is both kidneys, single, or prevalent kidney. The best indicator of such an effect on global residual renal mass is a rapid rate of decline of renal function and appears to be the most reliable criteria used to identify functional renal ischemia which is associated with a favorable response to renal revascularization. Further prospective, randomized studies with larger sample of patients are needed to validate our data and permit more selected evidence-based criteria other than anatomical to be used to select patients with ARAS for angioplasty and renal artery stenting.
ACKNOWLEDGMENT
The authors acknowledge Anatole Besarab, MD, Henry Ford Health System, Detroit, MI, for clinical review of the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: A systemic literature review. J Hypertens. 2009;27:1333–1340.
- Caps MT, Perissinotto C, Zierler RE, . Prospective study of atherosclerotic disease progression in the renal artery. Circulation 1998;98:2866–2872.
- Van Jaarsveld BC, Krijnen P, Pieterman H, . The effect of balloon angioplasty on hypertension in atherosclerotic renal artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med. 2000;342:1007–1014.
- Bax L, Woittiez AJ, Kouwenberg HJ, . Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: A randomized trial. Ann Intern Med. 2009;150:840–848.
- Wheatley K, Ives N, Gray R, . Revascularization versus medical therapy for renal artery stenosis. N Engl J Med. 2009;361:1953–1962.
- Hoffman U, Edwards JM, Carter S, . Role of duplex scanning for the detection of atherosclerotic renal artery stenosis. Kidney Int. 1991;39:1232–1239.
- Stavros AT, Parker SH, Yakes WF, . Segmental renal artery tardus parvus waveform abnormalities pattern recognition approach to duplex sonographic diagnosis of renal artery stenosis. Radiology 1992;184:487–492.
- Levey AS, Coresh J, Greene T, . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254.
- White CJ, Olin J. Diagnosis and management of atherosclerotic renal artery stenosis: Improving patient selection and outcomes. Nat Clin Pract. 2008;6:176–190.
- Weinberg MD, Olin JW. Stenting for atherosclerotic renal artery stenosis: One poorly designed trial after another. Cleve Clin J Med. 2010;77:164–171.
- Beutler JJ, Van Ampting JM, Van De Ven PJ, . Long term effects of renal stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol. 2001;12:1475–1481.
- Textor SC. Revascularization in atherosclerotic renal artery disease. Kidney Int. 1998;53:799–811.
- Harden PN, MacLeod MJ, Rodger RSC, . Effect of renal artery stenting on progression of renovascular renal failure. Lancet 1997;349:1133–1136.
- Dorros G, Jaff M, Mathiak L, . Four year follow up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 1998;98:642–647.
- Ramos F, Kotliar C, Alvarez D, . Renal function and outcome of PTRA and stenting for atherosclerotic renovascular disease. Kidney Int. 2003;63:276–282.
- Granerus G, Aurell G. References values for 51 CR-EDTA clearance as measure of glomerular filtration rate. Scand J Clin Invest. 1981;41:611–616.
- Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation 2005;112:1362–1374.
- Radermacher J, Weinkove R, Haller H. Techniques for predicting favourable response to renal angioplasty in patients with renovascular disease. Curr Opin Nephrol Hypertens. 2002;10:799–805.
- Garcia-Criado A, Gilabert R, Nicolau C, . Value of Doppler sonography for predicting clinical outcome after renal artery revascularization in atherosclerotic renal artery stenosis. J Ultrasound Med. 2005;24:1641–1647.
- Radermacher J, Chavan A, Bleck J, . Use of ultrasonography to predict the outcome of therapy for renal artery stenosis. N Engl J Med. 2001;344:410–417.
- Wright JR, Shurrab AE, Cheung C, . A prospective study of the determinants of renal function outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis. 2002;39:1153–1161.
- Muray S, Martin M, Amoedeo ML, . Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis. 2002;39:60–66.
- Baboolal K, Evans C, Moore RH. Incidence of end stage renal disease in medically treated patients with severe bilateral atherosclerotic renovascular disease. Am J Kidney Dis. 1998;31:971–977.
- Webster J, Marshall F, Abdalla M, . Randomized comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens. 1998;12:329–335.
- Plouin PF, Chatellier G, Darne B, . Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: A randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998;31:823–829.