Abstract
Background: The mortality rates of end-stage renal disease patients have significantly declined over the past decade. However, there are few reports on the risk factors for mortality in stable peritoneal dialysis (PD) patients who survive for a considerable time. Patients and methods: We reviewed the medical records and identified all adult patients who received PD between April 2001 and March 2009 in our institution. The total cohort was 550 patients. Among these patients, 383 patients were enrolled as stable PD patients. Results: The cumulative survival of the stable PD patients was 91.6% at 3 years and 78.7% at 5 years. On univariate analysis, old age (≥65 years of age), hypoalbuminemia (<35 g/L), log C-reactive protein (CRP) (≥0.84), phosphorus (<1.13 mmol/L), statin, icodextrin, and the Davies index were associated with mortality for all PD patients. Old age, hypoalbuminemia, log CRP, phosphorus, the residual renal function (RRF) (≤4 mL/min/1.73 m2) at 24 months, renin–angiotensin system blockade, icodextrin, and the Davies index were associated with mortality for the stable PD patients. Multivariate analysis showed that, among the variables, age, log CRP, phosphorus, initial RRF, and the Davies index were associated with mortality for all PD patients. In stable PD patients, age, log CRP, phosphorus, RRF at 24 months, and the Davies index were associated with mortality. Conclusion: Initial high RRF combined with the RRF preservation, maintenance of proper phosphorus, control of inflammation, and proper management of comorbidities may help to improve the survival of PD patients including stable PD patients.
BACKGROUND
Peritoneal dialysis (PD) is an established renal replacement therapy for patients with end-stage renal disease (ESRD). The incidence and prevalence of ESRD continues to increase worldwide. Accordingly, the number of patients on PD has been increasing.Citation1
The survival rate of PD patients is less as compared with that of the general population. Age, gender, underlying disease, the residual renal function (RRF), and infection are the classical independent predictors of PD patient survival. However, improved care measures, such as preservation of the RRF, peritoneal membrane integrity, and control of infection, have resulted in a growing population of long-term survivors.Citation1,2 According to the ANZDATA Registry, the 3-year survival rate was 54% in 1994–1996 and 64% in 2003–2005.Citation3 Thus, patient survival has gradually improved with time. However, most of the previous studies have included patients with a risk of early death.Citation1–3 There are few reports on the risk factors for stable PD patients who survive for a considerable time. This study addressed this issue.
PATIENTS AND METHODS
We reviewed the medical records at Yeungnam University Hospital in Korea and identified 550 adult (>18 years of age) patients who received continuous ambulatory peritoneal dialysis between January 1996 and April 2011. Among these patients, those who maintained PD for more than 2 years were defined as stable PD patients (n = 383). This study was approved by the Institutional Review Board of Yeungnam University Hospital (PCR11-45). Informed consent was waived by the board.
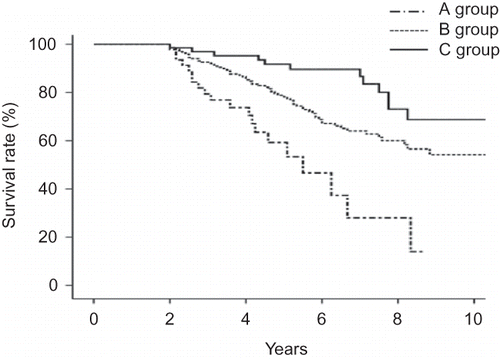
The clinical and laboratory data collected 1 month after the initiation of PD included age, gender, body surface area (m2), body mass index (BMI) (kg/m2), level of hemoglobin (g/L), albumin (g/L), C-reactive protein (CRP; normal: <5 mg/L), RRF (mL/min/1.73 m2), normalized protein equivalent of nitrogen appearance (nPNA) (g/kg/day), serum calcium (mmol/L), phosphorus (mmol/L), intact-parathyroid hormone (i-PTH, ng/L), mean arterial pressure (MAP; mmHg), and the weekly Kt/V, and peritoneal equilibration tests. The laboratory findings were measured every 6 months after the initiation of PD. The mean value of the laboratory findings during the follow-up was used as an indicator of the time-averaged (TA) data. The RRF was calculated from the collection of 24 h urine samples as the average of the renal creatinine and urea clearances. MAP was calculated by [(2× diastolic blood pressure) + systolic blood pressure] divided by 3. At the initiation of PD, the comorbidities were graded by the Davies index.Citation4 The comorbidities by the Davies index were ischemic heart disease, peripheral vascular disease, left ventricular dysfunction, diabetes mellitus (DM), systemic collagen vascular disease, and other significant pathologies. As described previously, the Davies index was categorized as low risk (0), intermediate risk (1–2), and high risk (≥3). The following data during follow-up were documented from the patient records: use of icodextrin, statin, renin–angiotensin system (RAS) blockade, and peritonitis. During the follow-up, the patients who received icodextrin, statin, or RAS blockade were defined as those treated for more than 1 year. The mortality data were collected from the initiation of PD. Stable PD patients were divided into three groups according to TA phosphorus: A group (<1.13 mmol/L), B group (1.13–1.78 mmol/L), and C group (>1.78 mmol/L).
The data were analyzed using SPSS version 17 (SPSS, Chicago, IL, USA). Continuous values are reported as mean ± SD. Categorical variables are expressed as counts and percentages. For continuous variables, means were compared using t-test and one-way analysis of variance. Because TA CRP distribution was skewed, log-transformed values were used in these analyses. The survival estimates were calculated according to Kaplan–Meier and Cox regression analyses. p-Values of <0.05 were considered to be statistically significant.
RESULTS
Patient Characteristics
Patient characteristics are shown in . The mean follow-up from the time of starting PD was 44.0 ± 36.4 months in all patients. The mean follow-up from the time of starting PD was 64.9 ± 31.3 months in stable PD patients. The causes of ESRD in all patients were DM in 277 patients (50.4%), hypertension (HTN) in 142 patients (25.8%), glomerulonephritis (GN) in 58 patients (10.5%), polycystic kidney disease in 11 patients (2.0%), others in 42 patients (7.6%), and an unknown cause in 20 patients (3.6%). The causes of ESRD in stable PD patients were DM in 154 patients (45.4%), HTN in 97 patients (28.6%), GN in 41 patients (12.1%), polycystic kidney disease in 8 patients (2.4%), others in 25 patients (7.4%), and an unknown cause in 14 patients (4.1%).
Table 1. Characteristics of PD patients.
In all patients, TA albumin was 37.7 ± 4.2 in the low-risk group, 34.4 ± 4.9 in the intermediate-risk group, and 33.1 ± 4.9 in the high-risk group (p < 0.001). In stable PD patients, TA albumin was 38.0 ± 4.0 in the low-risk group, 35.1 ± 4.3 in the intermediate-risk group, and 33.9 ± 5.0 in the high-risk group (p < 0.001). TA albumin was decreased in two groups as the comorbidity index increased.
TA RRF was 2.9 ± 3.1 in all patients and 2.6 ± 3.2 in stable PD patients. In stable PD patients, TA MAP was 97.8 ± 8.8 in RRF < 4 at 24 months and 95.7 ± 6.6 in RRF > 4 (p = 0.068), respectively. RRF at 24 months was 1.5 ± 0.5 in A group, 1.2 ± 0.4 in B group, and 1.0 ± 0.2 in C group (p < 0.001) (). TA i-PTH was 105.6 ± 108.4 in A group, 182.3 ± 172.6 in B group, and 217.3 ± 240.5 in C group (p = 0.016). TA albumin was 34.0 ± 6.0 in A group, 36.2 ± 4.2 in B group, and 37.0 ± 3.8 in C group (p = 0.002). TA BMI was 23.7 ± 3.4 in A group, 24.2 ± 3.3 in B group, and 24.4 ± 3.9 in C group (p = 0.580). The grade of TA phosphorus increased as RRF at 24 months was decreased. Additionally, TA i-PTH and albumin were increased as the grade of TA phosphorus increased.
Table 2. Clinical and laboratory findings in stable PD patients according to phosphorus.
Survival Analysis
The cumulative survival of all patients was 75.2% at 3 years and 64.6% at 5 years. The cumulative survival of the stable PD patients was 91.6% at 3 years and 78.7% at 5 years. On univariate analysis, old age (≥65 years of age), hypoalbuminemia (<35 g/L), log CRP (≥0.84), phosphorus (<1.13 mmol/L), statin, icodextrin, and the Davies index were associated with mortality for all patients (). Old age (≥65 years of age), hypoalbuminemia (<35 g/L), log CRP (≥0.84), phosphorus (<1.13 mmol/L), RRF (≤4 mL/min/1.73 m2) at 24 months, RAS blockade, icodextrin, and the Davies index were associated with mortality for the stable PD patients.
Table 3. Predictive factors affecting the mortality of patients on PD by univariate analysis.
Multivariate analysis showed that, among the variables, age, log CRP, phosphorus, initial RRF, and the Davies index were associated with mortality for all patients (). In stable PD patients, age, log CRP, phosphorus, RRF at 24 months, and the Davies index were associated with mortality. For all patients, the survival rate by the Davies index showed that the 5-year survival rate from the time of starting PD was 85.5% in the low-risk group, 58.9% in the intermediate-risk group, and 28.1% in the high-risk group (p < 0.001). For stable PD patients, the 5-year survival rate from the time of starting PD was 91.9% in the low-risk group, 74.0% in the intermediate-risk group, and 46.2% in the high-risk group (p < 0.001).
Table 4. Predictive factors affecting the mortality of patients on PD by multivariate analysis.
According to the level of phosphorus, the 5-year survival rate was 59.3% in A group, 78.1% in B group, and 91.7% in C group (p = 0.000) (). Totally, 183 patients died during the study period. Cardiovascular disease (35.5%) was the most common cause of death, followed by infection (29.1%), and cerebrovascular accident (6.4%).
DISCUSSION
Most of the previous studies published the survival analyses for the total follow-up period. The aim of this retrospective study was to evaluate the risk factors for mortality in stable PD patients. The main cause of ESRD in our patients was DM, followed by HTN. Age >65 years, a high Davies index, hypophosphatemia (<1.13 mmol/L), high log CRP, and RRF ≤4 mL/min/1.73 m2 were independent risk factors for mortality in all patients and in stable PD patients.
Early death by acute diseases should be excluded for a proper survival analysis. Many of the previous studies on survival have analyzed patients who survived the first 3–6 months after the start of dialysis.Citation5–7 However, these previous studies had several limitations. The analyses of the patients who survived for more than 3–6 months after the initiation of dialysis have not been consistent in excluding the effects of acute conditions at the start of dialysis. In addition, the initial findings at a short duration (≤3–6 months) make it difficult to predict long-term survival. Abnormal findings over the long term are more important than those at one point. The data from the 2004 Annual Dialysis Report indicate that the mortality rates have fallen for patients on dialysis for under 2 years, but the mortality rates for those patients on dialysis for more than 2 years have remained relatively stable.Citation8 These data show those mortality rates for those patients on dialysis for <2 years may not be associated with conditions that develop over time. To overcome these limitations, this study analyzed all patients who underwent PD and reanalyzed for stable patients who survived and maintained PD for over 2 years. The average of the findings for each 6 months is given as the TA variables.
The survival of elderly patients on PD is generally shorter than the survival of younger patients on PD.Citation9–13 The result of this study is similar to that of the previous results. The elderly patients on PD present with important morbidity, which often makes it difficult to indicate the start of PD.Citation14 Over the past 10 years, the rates of patients with ESRD have risen most quickly among the elderly.Citation2 Li et al.Citation15 observed no significant difference of the mortality between the elderly group and the nonelderly group. Old age as a risk factor for decreased survival may have changed due to the development of better medical care and improved survival in elderly patients. These circumstances have generated a growing interest in this particular patient population.
High comorbidity is a well-defined predictor of the outcomes of PD patients.Citation4,16,17 Patients on PD have received considerable attention due to the association of PD with a number of comorbidities, including viral hepatitis, coronary heart disease, DM, and HTN. Therefore, the comorbidity indexes as a predictor of mortality are more important than a single comorbidity. The Davies comorbid index was calculated for each patient as the total number of each patient’s comorbid conditions.Citation4 We analyzed the survival rate of patients according to the Davies index, which was categorized as low, intermediate, and high risk. Multivariate analysis revealed that as the Davies index becomes higher, PD patients tended to show lower survival. This study shows that the comorbid indexes are independent predictors of early death as well as mortality in the stable PD patients. Steady correction of comorbidities may help to improve survival of the stable PD patients.
Preservation of RRF has been well documented as a single predictor of PD patient survival.Citation18–23 The benefits of RRF in dialysis patients are maintenance of euvolemia, improved blood pressure control, superior phosphate control, and removal of uremic toxin.Citation21 This study supports the importance of preserving RRF of all patients including stable PD patients. In previous studies, preservation of RRF was defined as a 10 L or greater weekly glomerular filtration rate or urine volume exceeding 250 mL/day.Citation18,21–23 Long-term preservation of RRF in our study was defined as more than 4 mL/min (the mean of the creatinine and urea clearances) at 24 months after the start of PD. There is a need for a single consensus definition for the preservation of RRF in the long-term survivors on PD.
The association between the preservation of RRF and the control of phosphorus and blood pressure has been recognized and was confirmed in some studies.Citation24,25 Our study shows that the preservation of RRF favors the control of phosphorus. There was a significant difference in RRF according to TA phosphorus group in the stable PD patients. The grade of TA phosphorus decreased as RRF was increased. Additionally, there was no significant difference in the MAP according to RRF, but the trend showed an association between the MAP and the preservation of RRF (97.8 ± 8.8 in patients with RRF < 4 and 95.7 ± 6.6 in patients with RRF > 4; p = 0.068).
The Kidney Disease Outcomes Quality Initiative (KDOQI) guideline recommends that the serum levels of phosphorus should be maintained between 1.13 and 1.78 mmol/L in dialysis patients.26 Our study shows that the survival rate in stable PD patients was improved as the grade of TA phosphorus was increased. High survival rate in patients with hyperphosphatemia may be associated with good nutritional status. Although hyperphosphatemia is associated with high mortality, high phosphorus may be related with good appetite. Serum albumin in the C group was higher than in the other groups. There was no significant difference in the BMI, but the trend showed that BMI was increased as the level of TA phosphorus increased. Additionally, C group had less comorbidities than other groups.
Malnutrition and inflammation are highly prevalent among PD patients. The association between these markers and mortality has been recognized for over a decade and this has been confirmed.Citation27–29 In this multivariate analysis, TA albumin was not associated with mortality for PD patients. Our data showed that the TA albumin decreased as the grade of the Davies index increased. Therefore, hypoalbuminemia in our study is more closely associated with secondary change according to the comorbidities than the two variables per se. Our data also showed that the TA CRP was associated with mortality in both all patients and stable PD patients. In this study, TA CRP was transformed to log TA CRP. High CRP group was defined as ≥0.84 in log TA CRP. This is based on the mean of log CRP in all patients. Studies using data with normal distribution will be needed to define the level of high CRP.
In conclusion, our results demonstrate that old age, hypophosphatemia, high CRP, a high comorbid condition, and low RRF are significantly correlated with the mortality of both stable PD patients and all PD patients. Risk factors for mortality are not different between all PD patients and stable PD patients. Therefore, initial high RRF combined with the RRF preservation, maintenance of proper phosphorus, control of inflammation, and proper management of comorbidities may help to improve the survival of PD patients including stable PD patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Kendrick J, Teitelbaum I. Strategies for improving long-term survival in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2010;5:1123–1131.
- US Renal Data System. USRDS 2009 Annual Data Report: Atlas of Chronic kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
- Johnson D, Chang S, Excell L, Livingston B, Bannister K, McDonald SP. Peritoneal dialysis. In: McDonald SP, Excell L, eds. ANZDATA Registry Report 2006. Adelaide, SA: Australian and New Zealand Dialysis and Transplant Registry; 2007:8–11.
- Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17:1085–1092.
- Chen KH, Lin JL, Lin-Tan DT, . Glycated hemoglobin predicts mortality in nondiabetic patients receiving chronic peritoneal dialysis. Am J Nephrol. 2010;32:567–574.
- Han SS, Ahn JM, Chin HJ, . Impact of C-reactive protein and pulse pressure evaluated at the start of peritoneal dialysis on cardiovascular events in the course of treatment with peritoneal dialysis. Perit Dial Int. 2010;30:300–310.
- Park JT, Chang TI, Kim DK, . Metabolic syndrome predicts mortality in non-diabetic patients on continuous peritoneal dialysis. Nephrol Dial Transplant. 2010;25:599–604.
- US Renal Data System. USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2004.
- De Vecchi AF, Maccario M, Braga M, Scalamogna A, Castelnovo C, Ponticelli C. Peritoneal dialysis in nondiabetic patients older than 70 years: Comparison with patients aged 40 to 60 years. Am J Kidney Dis. 1998;31:479–490.
- Winkelmayer WC, Glynn RJ, Mittleman MA, Levin R, Pliskin JS, Avorn J. Comparing mortality of elderly patients on hemodialysis versus peritoneal dialysis: A propensity score approach. J Am Soc Nephrol. 2002;13:2353–2362.
- Kadambi P, Troidle L, Gorban-Brennan N, Kliger AS, Finkelstein FO. APD in the elderly. Semin Dial. 2002;15:430–433.
- Yang X, Fang W, Kothari J, . Clinical outcomes of elderly patients undergoing chronic peritoneal dialysis: Experiences from one center and a review of the literature. Int Urol Nephrol. 2007;39:1295–1302.
- Catizone L, Malacarne F, Bortot A, . Renal replacement therapy in elderly patients: Peritoneal dialysis. J Nephrol. 2010;23:S90–S97.
- Murtagh F, Marsh J, Donohoe P, Ekbal N, Sheerin N, Harris F. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22:1995–1962.
- Li PK, Law MC, Chow KM, . Good patient and technique survival in elderly patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2007;27:S196–S201.
- Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37:337–342.
- Miskulin DC, Meyer KB, Martin AA, . Choices for healthy outcomes in caring for end-stage renal disease (CHOICE) study. Comorbidity and its change predict survival in incident dialysis patients. Am J Kidney Dis. 2003;41:149–161.
- Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162.
- Shemin D, Bostom AG, Pastan S, Laiberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis. Am J Kidney Dis. 2001;38:85–90.
- Termorshuizen F, Korevaar JC, Dekker FW, . The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: An analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis. 2003;41:1293–1302.
- Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: A critical review. Am J Kidney Dis. 2009;53:1068–1081.
- Maiorca R, Brunori G, Zubani R, . Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant. 1995;10:2295–2305.
- Liao CT, Chen YM, Shiao CC, . Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant. 2009;24:2909–2914.
- Wang AY, Woo J, Wang M, . Important differentiation of factors that predict outcome in peritoneal dialysis patients with different degrees of residual renal function. Nephrol Dial Transplant. 2005;20:396–403.
- Menon MK, Naimark DM, Bargman JM, Vas SI, Dreopoulos DG. Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant. 2001;16:2207–2213.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl.3):S1–S201.
- Avram MM, Mittman N, Bonomini L, Chattopadhyay J, Fein P. Markers for survival in dialysis: A seven-year prospective study. Am J Kidney Dis. 1995;26:209–219.
- Avram MM, Bonomini LV, Sreedhara R, Mittman N. Predictive value of nutritional markers (albumin, creatinine, cholesterol, and hematocrit) for patients on dialysis for up to 30 years. Am J Kidney Dis. 1996;28:910–917.
- Owen WF, Lowrie EG. C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int. 1998;54:627–636.