Abstract
Reversible posterior leukoencephalopathy syndrome (RPLS) is characterized by headache, altered consciousness, seizures, and cortical blindness. The most frequent etiological factors are hypertension, kidney diseases, and immunosuppressive drugs such as steroids and cyclophosphamide. Herein we present a case of a 22-year-old female patient presented with alveolar hemorrhage and acute renal failure necessitating hemodialysis. In renal biopsy, necrotizing crescentic glomerulonephritis and immunofluorescence pattern compatible with Goodpasture syndrome were found. Anti-glomerular basement membrane antibody result was positive. At follow-up, respiratory failure ensued, steroid pulse treatment was started, and she was transferred to intensive care unit (ICU). In the ICU, she had visual disturbances and blindness together with seizures. Cranial magnetic resonance imaging (MRI) revealed irregular T2- and fluid-attenuated inversion recovery (FLAIR)-weighted lesions in bilateral occipital lobes. On clinical and radiological grounds, RPLS was diagnosed. With the supportive and anti-hypertensive treatment, RPLS was resolved without a sequela. Subsequent cranial MRI was totally normal. In the literature, RPLS associated with Goodpasture syndrome was reported only once. Hypertension and methylprednisolone might be the responsible etiologies in this case.
INTRODUCTION
Reversible posterior leukoencephalopathy syndrome (RPLS) is characterized by headache, altered mental functioning, seizures and visual loss, including cortical blindness in association with usually reversible bilateral posterior hemispheric edema on neuroimaging. Association of RPLS with connective tissue disorders such as systemic lupus erythematosus (SLE) is well known but Goodpasture syndrome has been reported to be related to RPLS only once in the literature. This is the second report revealing such possible relationship.
CASE PRESENTATION
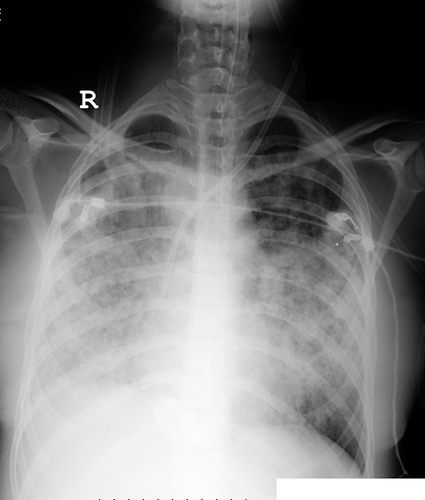
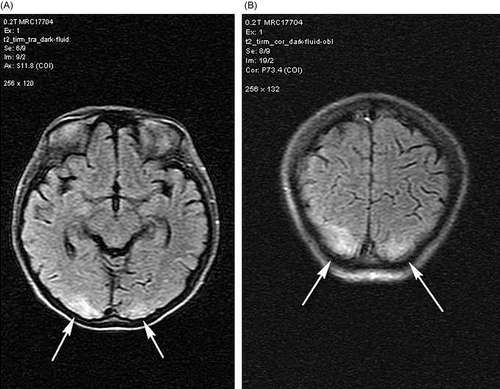
A 22-year-old female presented with alveolar hemorrhage () and acute renal failure necessitating hemodialysis. Basic laboratory results were as follows: serum creatinine, 9.5 mg/ dL; total protein, 5.9 g/dL; albumin, 3.3 g/dL; erythrocyte sedimentation rate, 61 mm/h; C-reactive protein, 5.5 mg/L; leukocyte, 12.200/uL; hemoglobin, 9.9 g/dL; and thrombocytes, 263.000/uL. In renal biopsy, necrotizing crescentic glomerulonephritis and immunofluorescence pattern compatible with Goodpasture syndrome were found. Anti-glomerular basement membrane antibody result was also positive. Pulse steroid and cyclophosphamide treatments were planned but after the first steroid pulse treatment (1 g/day), respiratory failure ensued, and she was transferred to intensive care unit (ICU). She was hypertensive despite ultrafiltration of about 2500 cc/day with daily hemodialysis treatment and anti-hypertensive drugs: amlodipine 10 mg and doxazosin 8 mg/day. Plasmapheresis was also performed eight times in ICU. At follow-up, she had visual disturbances and blindness together with seizures. Cranial magnetic resonance imaging (MRI) revealed irregular T2- and fluid-attenuated inversion recovery (FLAIR)-weighted lesions in bilateral occipital lobes (). On clinical and radiological grounds, RPLS was diagnosed. With thrice weekly hemodialysis and anti-hypertensive treatment with amlodipine and doxazosin, RPLS was resolved without a sequela in about 2 weeks. Subsequent cranial MRI was totally normal. At follow-up the general status of the patient improved extubated, and she was discharged from the ICU to nephrology clinic at the end of second week of intensive care. The patient’s treatment was continued with monthly pulse cyclophosphamide (750 mg) followed by tapering doses of oral maintenance methylprednisolone. Eventually the patient received 3 g of pulse methylprednisolone and subsequent tapering doses of 1 mg/kg/day prednisolone. Unfortunately, renal functions did not improve and the patient was totally anuric needing hemodialysis treatment; however, she had no respiratory complaints.
DISCUSSION
RPLS is characterized by headache, altered consciousness, seizures, and cortical blindness.Citation1 The most frequent etiological factors are hypertension, acute/chronic kidney disease, uremia, eclampsia, organ transplantation, connective tissue diseases, and immunosuppressive agents such as steroids and cyclophosphamide. Severe hypertension with autoregulatory failure, hyperperfusion, and vasodilatation, resulting in vasogenic edema, is often considered to be the underlying mechanism.Citation2 Vasculopathy (either vasoconstriction or vasodilatation), abnormal cerebral perfusion (hypo- or hyper-perfusion), and vasogenic brain edema are supposed to be the parts of pathophysiology of RPLS.3 Additionally immunosuppressive therapy combined with uremia may be hypothesized to have neurotoxic effects on the central nervous system.Citation4 However, these hypotheses are not comprehensive and the pathogenesis of RPLS needs further clarification.
MRI is essential as a diagnostic tool in RPLS in which typical findings are white matter edema in the posterior regions of the cerebral hemispheres, often symmetrical involvement of the parietal and occipital lobes.Citation4,5 These abnormalities can be demonstrated using T2 and FLAIR sequences.Citation5,6 With treatment, the clinical and MRI abnormalities partially or completely resolve in 2–3 weeks.Citation4 This syndrome is often benign, but death has been reported.Citation7 Association of RPLS with connective tissue disorders is well known, especially with SLE. It seems that the occurrence of SLE-associated RPLS was basically caused by hypertension induced by fluid retention secondary to acute renal failure and a high dose of corticosteroids.3 However, in the literature, RPLS associated with Goodpasture syndrome was reported only once, which was considered to be related to cyclophosphamide treatment. Our patient did not receive cyclophosphamide before the diagnosis of RPLS. Hypertension and methylprednisolone were probably the responsible etiologies in our case. In the management of the connective tissue disorders with renal involvement, control of hypertension is of paramount importance to prevent RPLS.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Lee VH, Wijdicks EF, Manno EM, . Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205–210.
- Schwartz RB. Hyperperfusion encephalopathies: Hypertensive encephalopathy and related conditions. Neurologist. 2002;8:22–34.
- Zhang YX, Liu JR, Ding MP, Reversible posterior encephalopathy syndrome in systemic lupus erythematosus and lupus nephritis. Intern Med. 2008;47(9):867–875.
- Hinchey J, Chaves C, Appignani B, . A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500.
- Mukherjee P, McKinstry RC. Reversible posterior leukoencephalopathy syndrome: Evaluation with diffusion-tensor MR imaging. Radiology. 2001;219:756.
- Provenzale JM, Petrella JR, Cruz LC, . Quantitative assessment of diffusion abnormalities in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2001;22:1455.
- Giménez-Mesa E, Martínez-Salio A, Porta-Etessam J, . Reversible posterior leukoencephalopathy in a patient with non-Hodgkin’s lymphoma treated with CHOP chemotherapy. An Med Interna. 2001;11:591–593.