Abstract
Background/aims: Necrostatin-1 (Nec-1) inhibits necroptosis, a nonapoptotic cell death pathway. Acute kidney injury (AKI) is a clinical problem of high incidence and mortality. It involves several mechanisms of cell death. We aim to evaluate the effect of Nec-1 in the toxic kidney injury model by cisplatin. Methods: We analyzed the effect of Nec-1 in AKI by cisplatin in human proximal tubule cells by flow cytometry. Results: Our results show that Nec-1 has no effect on apoptosis in renal tubular epithelial cells (Nec-1 + Cis group 13.4 ± 1.7% vs. Cis group 14.6 ± 1.4%) (p > 0.05). But, in conditions in which apoptosis was blocked by benzyloxy-carbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk) the use of Nec-1 completely reversed cell viability (Nec-1 + Cis + z-VAD group 72.9 ± 6.3% vs. Cis group 35.5 ± 2.2%) (p < 0.05) suggesting that Nec-1 has effect on nonapoptotic cell death (necroptosis). Conclusion: Our findings suggest that the combined use of apoptosis and necroptosis inhibitors can provide additional cytoprotection in AKI. Furthermore, this is the first study to demonstrate that Nec-1 inhibits tubular kidney cell death and restores cell viability via a nonapoptotic mechanism.
INTRODUCTION
Acute kidney injury (AKI) is a common clinical problem associated with high mortality.Citation1 Studies have shown that apoptotic and necrotic cell death are often found in AKI caused by nephrotoxic injury.Citation2,3
Apoptosis is a type of cell death that is caspase dependent. The caspases are a family of proteases that play a key role in the execution of apoptosis. Thus, inhibition of caspases during apoptotic cell death can block apoptosis.Citation4
Recently, Degterev et al.Citation5 identified a novel type of cell death called necroptosis—a regulated caspase-independent cell death mechanism with morphological features resembling necrosis—which occurs when apoptosis is blocked by caspase inhibitors. Importantly, they also identified a specific necroptosis inhibitor, necrostatin-1 (Nec-1).Citation5 Experimental studies using different models have shown that blocking both cell death pathways, namely, apoptosis and necroptosis, by caspase inhibitors and Nec-1, respectively, was effective as combination therapy.Citation5–11
Thus, the use of inhibitors of apoptosis and necroptosis may result in additive protective effect in AKI. In this study, we hypothesized that inhibition of apoptosis triggers necroptosis in human proximal kidney cells (HK-2).
Our aims were to evaluate the effect of Nec-1, which blocks a critical step in necroptosis, in the toxic kidney injury model by cisplatin and to evaluate the cytoprotective treatment by combined therapy (anti-apoptotic and anti-necroptotic).
METHODS
Reagents
The following reagents were used in this study: cis-platinum(II)-diamine dichloride (cisplatin, Cis; Sigma, St. Louis, MO, USA); necroptosis inhibitor, Nec-1 (Sigma); general pan-caspase inhibitor, benzyloxy-carbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk; Becton Dickinson, Franklin Lakes, NJ, USA); and bisbenzimide HOE 33342 [(2’-[4-ethoxyphenyl]-5-[4-methyl-1-piperazinyl]-2,5’-bi-1H-benzimidazole)] trihydrochloride dye (Hoechst; Sigma).
Cell Culture
HK-2 (human proximal tubule cells) cells were obtained from ATCC (Manassas, VA, USA) and maintained in a mixture of Ham’s F12 and Dulbecco’s modified Eagle’s medium (DMEM:F12; Life Technologies, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Life Technologies), 50 units penicillin G, and 6 mg streptomycin/mL (Life Technologies) at 37°C in 5% CO2 atmosphere. Experiments were performed with cells grown to 80% confluence.
Cell Treatment
Cells were divided into seven groups as follows: (1) Control group (n = 6): cells were maintained in DMEM:F12 supplemented with 10% FBS for 18 h; (2) Cis group (n = 6): cells were treated with 100 μM of cisplatin for 18 h; (3) Nec-1 + Cis group (n = 6): cells were pretreated with 50 μM of Nec-1 for 6 h. Nec-1 was removed after this time and the cells were washed twice with phosphate-buffered saline (PBS) and treated with 100 μM of cisplatin for 18 h; (4) Cis + z-VAD group (n = 6): cells were treated with 100 μM of cisplatin and 50 μM of z-VAD-fmk for 18 h; (5) Nec-1 + Cis + z-VAD group (n = 6): cells were pretreated with 50 μM of Nec-1 for 6 h. Nec-1 was removed after this time and the cells were washed twice with PBS and treated with 100 μM of cisplatin and 50 μM of z-VAD-fmk for 18 h; (6) z-VAD group (n = 6): cells were treated with 50 μM of z-VAD-fmk for 18 h; and (7) Nec-1 group (n = 6): cells were treated with 50 μM of Nec-1 for 6 h. Nec-1 was removed after this time and the cells were washed twice with PBS and maintained in DMEM:F12 supplemented with 10% FBS for 18 h.
Cell Viability and Apoptosis
To evaluate viability and apoptosis after treatment the cells were washed with PBS and 2 × 105 cells were transferred to each tube with 5 μL Annexin V-FITC (Becton Dickinson), 4 μL of propidium iodide (PI; Becton Dickinson), and 400 μL of binding buffer (Becton Dickinson). The cells were then incubated for 20 min at room temperature in the dark. The following controls were used: unstained cells, cells stained with Annexin V-FITC (no PI), cells stained with PI (no Annexin V-FITC), and cells stained with Annexin V-FITC/PI. The degree of viability and apoptosis was assessed by flow cytometry (FACSCanto analyzer; Becton Dickinson Immunocytometry Systems). Cells staining positive for PI were considered as dead cells (necrosis or late apoptosis), cells staining positive only for Annexin V-FITC were considered as apoptotic cells, and cells staining negative for both PI and Annexin V-FITC were considered as viable cells.
Fluorescent Microscopy
HK-2 cells were washed with PBS three times, centrifuged, resuspended in PBS, and incubated with 10 μL of Hoechst solution (1.0 mM) in dark chamber at room temperature for 10 min. Cells were viewed by fluorescence microscopy using a UV filter. Apoptotic cells exhibit intense blue fluorescence.
Statistical Analyses
The results expressed as mean ± SD were used in cell culture experiments, where cell numbers were large enough to assume normal distribution. Statistical significance for differences among groups was tested by one-way ANOVA, followed by Tukey’s tests for multiple comparisons. The value of p < 0.05 was considered as statistically significant.
RESULTS
z-VAD-fmk and Nec-1 Inhibit Cell Death in HK-2 Cells
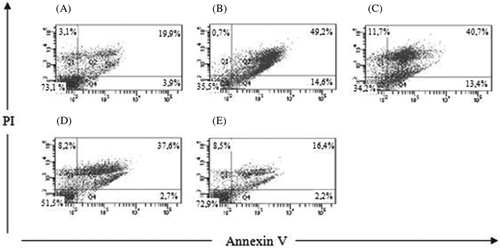
Kidney injury was induced by 100 μM of cisplatin in HK-2 cells after 18 h of treatment. shows the apoptotic cells with Hoechst staining. A–E represents the Control group, Cis group, Nec-1 + Cis group, Cis + z-VAD group, and Nec-1 + Cis + z-VAD group, respectively. Apoptotic cells stained with Hoechst appear as intense blue fluorescence. A significant increase in the percentage of apoptosis was observed between the Control group and the Cis group, 3.9 ± 0.6% and 14.6 ± 1.4%, respectively (p < 0.05) (). The use of inhibitors z-VAD-fmk (50 μM) alone or Nec-1 (50 μM) alone had no effect on cell death (4.5 ± 1.6% and 5.6 ± 0.6%, respectively) (p > 0.05). The Nec-1 pretreatment did not protect cells from apoptosis caused by cisplatin. The Nec-1 + Cis group showed 13.4 ± 1.7% of apoptotic cells, while the Cis group showed 14.6 ± 1.4% (p > 0.05) (). As expected, the pan-caspase inhibitor blocked the apoptosis. shows that apoptosis in the Cis + z-VAD group (2.7 ± 0.6%) was significantly lower than the Cis group (14.6 ± 1.4%) (p < 0.05). The concomitant use of z-VAD-fmk and Nec-1 inhibited cell death, as shown by the Nec-1 + Cis + z-VAD group (2.2 ± 0.8%) versus Cis group (3.9 ± 0.6%) (p < 0.05) and Nec-1 + Cis + z-VAD group (2.2 ± 0.8%) versus Cis + z-VAD group (2.7 ± 0.6%) (p > 0.05). The percentage of apoptosis in Nec-1 + Cis group (13.4 ± 1.7%) shows that Nec-1 had no effect in the apoptotic cell death while the percentage of apoptotic cells in the Cis + z-VAD group (2.7 ± 0.6%) shows the ability of z-VAD to block the apoptosis (p < 0.05 for Nec-1 + Cis group vs. Cis + z-VAD group).
Figure 1. Representative fluorescence microscopy images of human proximal tubule cells (HK-2): (A) Control group, (B) Cis group, (C) Nec-1 + Cis group, (D) Cis + z-VAD group, and (E) Nec-1 + Cis + z-VAD group.
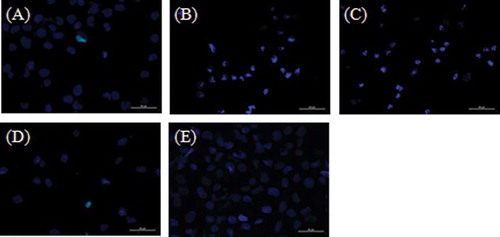
Figure 2. Protective effects of inhibitors [necrostatin-1 (Nec-1) and benzyloxy-carbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk)] on apoptosis induced by cisplatin in human proximal tubule cells (HK-2).Note: *p < 0.05, Cis group and Nec-1 + Cis group versus Control group, #p < 0.05, Cis group and Nec-1 + Cis group versus Nec-1 + Cis + z-VAD group.
![Figure 2. Protective effects of inhibitors [necrostatin-1 (Nec-1) and benzyloxy-carbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk)] on apoptosis induced by cisplatin in human proximal tubule cells (HK-2).Note: *p < 0.05, Cis group and Nec-1 + Cis group versus Control group, #p < 0.05, Cis group and Nec-1 + Cis group versus Nec-1 + Cis + z-VAD group.](/cms/asset/43c9deec-b186-4915-8f5d-40aa2d7510a2/irnf_a_647343_f0002_b.gif)
Additive Protective Effect of Inhibitors on Cell Death
The pretreatment with Nec-1 (50 μM) alone or z-VAD-fmk (50 μM) alone had no effect on cell viability (65.8 ± 1.7% and 63.8 ± 3.3%, respectively). We can see this by comparing Nec-1 group (65.8 ± 1.7%) with Control group (73.1 ± 1.7%) (p > 0.05); z-VAD-fmk group (63.8 ± 3.3%) with Control group (73.1 ± 1.7%) (p > 0.05); and Nec-1 group (65.8 ± 1.7%) with z-VAD-fmk group (63.8 ± 3.3%) (p > 0.05). In we show that cisplatin treatment significantly decreased cell viability from 73.1 ± 1.7% (Control group) to 35.5 ± 2.2% (Cis group) (p < 0.05). The combination of Nec-1 and cisplatin significantly decreased cell viability from 73.1 ± 1.7% (Control group) to 34.2 ± 5.2% (Nec-1 + Cis group) (p < 0.05) (). We can see a difference in viability between the Cis + z-VAD group and the Control group (51.5 ± 10.4% and 73.1 ± 1.7%, respectively) (p < 0.05) (). And, we did not observe difference in cell viability between the Control group (73.1 ± 1.7%) and the Nec-1 + Cis + z-VAD group (72.9 ± 6.3%) (p > 0.05), as shown in .
Figure 3. The effects of inhibitors [necrostatin-1 (Nec-1) and benzyloxy-carbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk)] on cell viability in human proximal tubule cells (HK-2) treated with cisplatin.Note: *p < 0.05, Cis group, Nec-1 + Cis group and Cis + z-VAD group versus Control group, #p < 0.05, Cis group, Nec-1 + Cis group and Cis + z-VAD group versus Nec-1 + Cis + z-VAD group.
![Figure 3. The effects of inhibitors [necrostatin-1 (Nec-1) and benzyloxy-carbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk)] on cell viability in human proximal tubule cells (HK-2) treated with cisplatin.Note: *p < 0.05, Cis group, Nec-1 + Cis group and Cis + z-VAD group versus Control group, #p < 0.05, Cis group, Nec-1 + Cis group and Cis + z-VAD group versus Nec-1 + Cis + z-VAD group.](/cms/asset/692fded7-6456-4396-a216-2501b274127a/irnf_a_647343_f0003_b.gif)
shows flow cytometry analysis of one sample of six studied in each group. The percentage represents the number of cells in each region with the region Q1 containing the necrotic population (Annexin V-negative/PI-positive), region Q2 containing the late apoptosis cells (double positive), region Q3 containing the viable population (double negative), and region Q4 containing the apoptotic population (Annexin V-positive/PI-negative). A–E represents the Control group, Cis group, Nec-1 + Cis group, Cis + z-VAD group, and Nec-1 + Cis + z-VAD group, respectively.
DISCUSSION
Our study shows that Nec-1—a specific inhibitor of necroptosis—increased cell viability in in vitro models of AKI. We investigated the efficacy of combined use of apoptosis and necroptosis inhibitors in human proximal kidney cells (HK-2).
AKI involves two mechanisms of cell death called necrosis and apoptosis. The morphological differences between these two types of cell death are listed in Apoptosis inhibition through the use of general pan-caspase inhibitor was widely used in several kidney injury models with moderate effectiveness in blocking cell death.Citation5–11,14–18 As we can see in the combined use of pan-caspase inhibitor and cisplatin (p < 0.05 for Cis + z-VAD group, 2.7 ± 0.6% vs. Cis group, 14.6 ± 1.4%) decreased apoptosis. However, cell viability was not completely reversed (Cis + z-VAD group, 51.5 ± 10.4% vs. Control group, 73.1 ± 1.7%, p > 0.05) () suggesting that the cell death in AKI can occur through several mechanisms.
Table 1. Morphological features of necrosis and apoptosis.
Recent studies show that in some models, such as cardiac and cerebral ischemia,Citation5–9 the apoptosis inhibition does not stop the process of cell death, activating nonapoptotic pathways, which would act as a cellular backup mechanism to eliminate damaged cells.Citation5
The necroptosis, identified by Degterev et al. Citation5, is a type of nonapoptotic cell death. It is a caspase-independent process, shows necrotic morphology, and can be blocked by a specific necroptosis inhibitor—Nec-1. Degterev et al. Citation5 showed that neuronal models that block both cell death pathways, namely, apoptosis and necroptosis, by caspase inhibitors and Nec-1 inhibitors, respectively, can provide additional neuroprotective benefits. Smith’s groupCitation9 have conducted studies in a model of myocardial ischemia-reperfusion injury (in vivo and in vitro), showing that Nec-1 has reduced infarct volume after middle cerebral artery occlusion. Other studies show that sitosterol-induced death in macrophages is caspase independent and can be blocked by Nec-1.Citation10,11 These studies demonstrate the effectiveness of Nec-1 in blocking nonapoptotic cell death in several in vivo and in vitro models. But, further research is needed to gain a greater understanding of the mechanism involved in necroptosis.
In this study we observe that Nec-1 has no effect on apoptosis in renal tubular epithelial cells, as shown in . The Nec-1 pretreatment did not protect cells from apoptosis induced by cisplatin, as we can see from the comparisons of viability rate between the Control group (73.1 ± 1.7%) and the Nec-1 + Cis group (34.2 ± 5.2%) (p < 0.05) and between the Nec-1 + Cis group (34.2 ± 5.2%) and the Cis group (35.5 ± 2.2%) (p > 0.05). These results are in agreement with data reported in previous studiesCitation5–8 showing that necroptosis is activated under conditions where apoptosis is inhibited. Thus, Nec-1, like a specific inhibitor of necroptosis, has effect only when apoptosis is blocked. The combined use of Nec-1 and general pan-caspase inhibitor (Nec-1 + Cis + z-VAD group, 72.9 ± 6.3%) allowed us to obtain cell viability above 20% compared with the group treated with cisplatin and z-VAD (Cis + z-VAD group, 51.5 ± 10.4%) (p < 0.05), as shown in . These findings suggest that the additional protective effect observed in the Nec-1 + Cis + z-VAD group is due to the action of Nec-1 on nonapoptotic cell death (necroptosis).
The incidence of AKI is between 0.7% and 1% in patients admitted to a general hospital and 30% in patients admitted to an intensive care unit.Citation19 Even with advances in treatment, the development of kidney injury is still associated with high mortality rates, ranging from 40% to 90%.Citation20,21 Thus, it is necessary to study new therapeutic strategies.
Our results show that the use of inhibitors of different cell death pathways in renal cells offers additive cytoprotection. In vivo model studies show that both z-VAD-fmkCitation22 and Nec-1Citation10 inhibitors have no toxic or side effects, suggesting that the combined use of these inhibitors could be a clinically useful tool. Furthermore, this is the first study to demonstrate that Nec-1 inhibits tubular kidney cell death and restores cell viability via a nonapoptotic mechanism (necroptosis).
In summary, necroptosis might be an important mode of cell death in kidney injury. The cytoprotection demonstrated with Nec-1 supports this fact. Finally, our results have potential relevance suggesting that the combined use of apoptosis and necroptosis inhibitors can provide additional cytoprotection in AKI. Nonetheless, further studies are needed to better understand the mechanisms by which necroptosis occurs in kidney injury.
ACKNOWLEDGMENTS
Funding. This work was supported by grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) N° 08/09773-4, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
This study was conducted at the Nephrology Division, Federal University of São Paulo – UNIFESP
REFERENCES
- Lines SW, Cherukuri A, Murdoch SD, Bellamy MC, Lewington AJ. The outcomes of critically ill patients with acute kidney injury receiving renal replacement therapy. Int J Artif Organs. 2011;34(1):2–9.
- Havasi A, Borkan S. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40.
- Kinsey GR, Okusa MD. Pathogenesis of acute kidney injury: Foundation for clinical practice. Am J Kidney Dis. 2011;58(2):291–301.
- Ueda N, Shah SV. Tubular cell damage in acute renal failure-apoptosis, necrosis, or both. Nephrol Dial Transplant. 2000;15(3):318–323.
- Degterev A, Huang Z, Boyce M, . Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119.
- Li Y, Yang X, Ma C, Qiao J, Zhang C. Necroptosis contributes to the NMDA-induced excitotoxicity in rat’s cultured cortical neurons. Neurosci Lett. 2008;447(2–3):120–123.
- Han W, Xie J, Li L, Liu Z, Hu X. Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis. Apoptosis 2009;14(5):674–686.
- Xu X, Chua KW, Chua CC, Liu CF, Hamdy RC, Chua BH. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res. 2010;1355:189–194.
- Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: A potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21(4):227–233.
- Bao L, Li Y, Deng SX, Landry D, Tabas I. Sitosterol-containing lipoproteins trigger free sterol-induced caspase-independent death in ACAT-competent macrophages. J Biol Chem. 2006;281(44):33635–33649.
- Tabas I. A two-carbon switch to sterol-induced autophagic death. Autophagy 2007;3(1):38–41.
- Vandenabeele P, Declercq W, Vanden Berghe T. Necrotic cell death and ‘necrostatins’: Now we can control cellular explosion. Trends Biochem Sci. 2008;33(8):352–355.
- Teng X, Degterev A, Jagtap P, . Structure-activity relationship study of novel necroptosis inhibitors. Bioorg Med Chem Lett. 2005;15(22):5039–5044.
- Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: Caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther. 2002;302(1):8–17.
- Sanz AB, Santamaría B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol. 2008;19(9):1634–1642.
- Galluzzi L, Kroemer G. Necroptosis: A specialized pathway of programmed necrosis. Cell 2008;135(7):1161–1163.
- Cummings BS, Kinsey GR, Bolchoz LJ, Schnellmann RG. Identification of caspase-independent apoptosis in epithelial and cancer cells. J Pharmacol Exp Ther. 2004;10(1):126–134.
- Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119(5):1275–1285.
- Moonen M, Fraipont V, Radermacher L, Masset C, Firre E, Warling X. Acute kidney injury: From concept to practice. Nephrol Ther. 2010; 7(3):172–177.
- Dirkes S. Acute kidney injury: Not just acute renal failure anymore? Crit Care Nurse. 2011;31(1):37–50.
- Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):1503–1520.
- Daemen MA, de Vries B, Buurman WA. Apoptosis and inflammation in renal reperfusion injury. Transplantation 2002;73(11):1693–1700.