Abstract
Introduction: Polyoma BK virus (BKV) has recently been identified to cause renal allograft dysfunction, which manifests as polyomavirus-associated nephropathy (PVAN). However, the presence and level of BKV DNA in renal allograft patients with good and stable renal function have remained undetermined. Methods: In this prospective study, serum samples were collected from a total of 45 renal allograft recipients with serum creatinine <155 μmol/L. In 17 patients, whose duration of transplantation was under 2 years, samples were collected at 3–4-month intervals for up to 2 years after transplantation. BK viral load was quantified using quantitative polymerase chain reaction (Q-PCR). Results: The BK viral load in asymptomatic renal allograft recipients was independent of the duration of transplantation and did not correlate with allograft function. The mean (± SD) level of viremia was 552.80 ± 1931.00 genome copies/mL, with 92.9% of patients having low levels of viremia corresponding to <1 × 103 copies/mL. In contrast, patients with proven PVAN had levels in the range of 106 copies/mL. Conclusions: The prevailing BK viral load in asymptomatic renal allograft patients is quantifiably low. Our findings may guide optimal immunosuppressive modulation in PVAN cases, where judicious manipulation of immunosuppression is required without inciting allograft rejection.
INTRODUCTION
The modern era of immunosuppression has significantly improved allograft survival in renal transplant patients by reducing rejection rates. However, this is paralleled by increased incidence and spectrum of infections posing as the main barrier to disease free survival after transplantation.Citation1
The human polyomavirus, BK virus (BKV), has recently surfaced as an important cause of allograft dysfunction manifesting as polyomavirus-associated nephropathy (PVAN) in renal transplant recipients.Citation2 BKV is a ubiquitous small DNA virus, ∼5300 bp in size, demonstrating a high seroprevalence in humans (>90%).Citation3 Primary infection, usually occurring in childhood via the oral and/or respiratory route, is innocuous but demonstrates latency by lying dormant in the uroepithelium and circulating leukocytes.Citation3 Asymptomatic intermittent viral replication with active shedding occurs in some immunocompetent individuals.Citation4
PVAN occurs in up to 10% of renal transplantation,Citation2,4 most commonly within the first 2 years.Citation5 It is caused by the reactivation of BKV infection in the allograft, which excites an interstitial nephritis,Citation6 and presents with clinically asymptomatic, slowly progressive allograft failure in 50–90% of cases.Citation4 The urinalysis is consistent with an interstitial nephritis with the passage of decoy cells, and definitive diagnosis requires tissue biopsy.Citation2,7 In severe cases, progressive irreversible damage amounts to subsequent graft loss.Citation5
BKV replication in the allograft has been correlated with the detection of BKV DNA in plasma by real-time quantitative polymerase chain reaction (Q-PCR),Citation8,11 and PVAN is associated with a serum BKV DNA level of ≥1 × 104 copies/mL.Citation2,7 The cornerstone of PVAN management involves the reduction of immunosuppression in an attempt to restrain viral replication without inciting acute rejection.Citation9,10 Therefore, BKV DNA levels can guide the optimal level of immunosuppressive reduction and serve as a quantifiable surrogate marker of the course of infection.Citation11
However, there appears to be limited data with regard to the presence and the level of BKV DNA in the plasma of renal transplant recipients without clinical evidence of PVAN. In this prospective study, BKV genomic copies in the plasma of renal transplant recipients with good and stable allograft function were quantified to establish the absolute background circulating levels in the absence of PVAN.
MATERIALS AND METHODS
Patient Selection
This study was approved by the University of Hong Kong Institutional Review Board. All participating subjects gave informed consent. Serum samples were collected from incident first-time kidney transplant recipients with serum creatinine <155 μmol/L. In addition, patients in whom the duration of transplantation was under 2 years were prospectively followed. For this subset of patients, serum samples were collected at 3–4-month intervals for up to 2 years after transplantation for determination of BKV DNA. Patients with allograft dysfunction, as defined by >35 μmol/L alteration in serum creatinine from baseline during the course of follow-up, were excluded.
Diagnostic Specimens
During follow-up, 5 mL of peripheral blood was collected from each patient at 3–4-monthly intervals for up to 2 years after transplantation. BKV DNA in the plasma (not whole blood) was extracted by the QIAamp DNA Blood Minikit (Qiagen, Basel, Switzerland) and eluted with 200 μL of buffer. Plasma was used to prevent concurrent BKV DNA extraction from circulating leukocytes.
PCR Primers and Probe
The PCR primers used for the BKV VP1 gene (GenBank accession number V01109) were (1) forward primer VPf (5′-AGT GGA TGG GCA GCC TAT GTA-3′, nucleotides 670–690) and (2) reverse primer VPr (5′-TCA TAT CTG GGT CCC CTG GA-3′, nucleotides 745–764). The TaqMan BKV probe (5′-AGG TAG AAG AGG TTA GGG TGT TTG ATG GCA CAG-3′, nucleotides 705–737) dual-labeled at the 5′ end with 6-carboxyfluorescein (FAM) and at the 3′ end with 6-carboxytetramethylrhodamine (TAMRA) was used. All were designed by Primer Express software (PE Biosystems, Foster City, CA, USA). Although VPf and VPr share substantial homology with another human polyomavirus, JC virus, the TaqMan probe does not, and therefore no interference was obtained during Q-PCR.Citation3
Quantitative PCR (Q-PCR)
Q-PCR was performed by a real-time PCR assay using the ABI Prism 7700 Sequence Detector (PE Biosystems). The PCR conditions have been previously documented.Citation12 Briefly, Q-PCR amplification reactions were set up in a reaction volume of 50 μL, using the TaqMan Universal PCR Master Mix (PE Biosystems) containing 10 μL of purified DNA, 200 and 400 nM of VPf and VPr, and 50 nM of TaqMan probe. Thermal cycling was initiated with a 2-min incubation at 50°C, followed by a first denaturation step of 10 min at 95°C and then 40 cycles of 95°C for 15 s (denaturation) and 60°C for 1 min (reannealing and extension). The copy number of target sequences can be deduced from the cycle thresholds.Citation13
Quantification of BKV
Standard curves for the quantification of BKV were constructed by plotting the cycle thresholds (CTs) against the logarithm of the starting amount of a serial dilution (0.5 fg to 5 pg) of the plasmid standard pB-VP1, which contained BKV VP1 gene sequence targeted by the Q-PCR.Citation2 Patient samples were tested in triplicate, their respective CTs were determined, and the initial starting sequence amount (genome equivalent copies) was calculated from the standard curve.Citation2
Quality Assurance
To ensure reliable result from the Q-PCR, universal bench work precautions were strictly adhered to. Cross-contamination during the procedure was prevented by the use of positive displacement pipettes with aerosol-resistant tips. This was further validated by using negative cassette blanks in each PCR. These did not yield positive results.
Plate-to-plate variations in PCR efficiencies were countered by constructing a standard curve in each PCR experiment by serial dilutions of the same stock solution of pB-VP1 at the start of the experiments. This ensured comparable results for analyses.
Statistical Analysis
Correlation of continuous data was assessed by Pearson’s product moment coefficient and comparison of categorical data was evaluated by one-way analysis of variance. p-Values of <0.05 were considered as statistically significant.
RESULTS
A total of 45 patients were recruited. One patient was excluded due to an elevation in serum creatinine of >35 μmol/L from baseline during the course of the study. Therefore, 44 patients were included in this analysis. The clinical characteristics of these 44 patients are shown in . Immunosuppressive regimens comprised of different combinations of prednisolone, cyclosporin A, tacrolimus, azathioprine, mycophenolate mofetil, and sirolimus. All patients had good and stable allograft function. This was defined by serum creatinine of <155 μmol/L with <35 μmol/L alteration from baseline, during the course of study, with no evidence of any decompensating clinicopathological entities such as graft rejection, obstruction, arterial stenosis, or urinary tract infection.
Table 1. Clinical characteristics of the study cohort (n = 44).
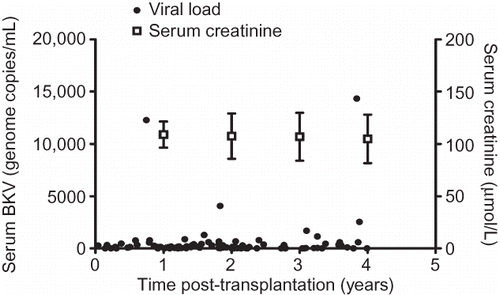
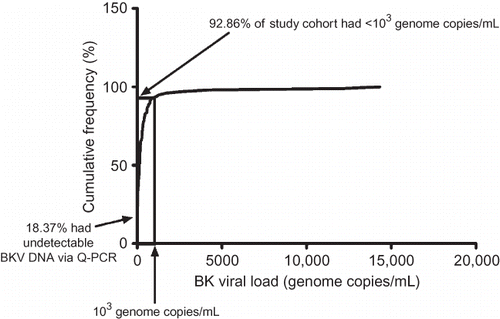
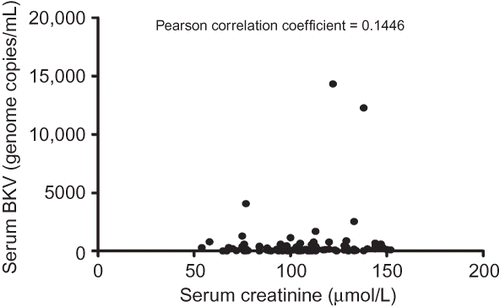
In our study, BK viral load in asymptomatic renal allograft patients, during the early post-transplantation period, was unrelated to time (). The mean level of viremia from all collected samples was 552.80 ± 1931.00 genome copies/mL, and the majority (92.9%) had low levels corresponding to <1 × 103 copies/mL. Furthermore, 18.4% of patients had undetectable circulating BKV DNA as measured by Q-PCR (). The corresponding mean level of serum creatinine was 104.70 ± 23.31 μmol/L, and there was no significant correlation between viral load and renal function ().
Figure 3. The quantitative relationship between BK viremia and renal allograft function in the early post-transplantation period.
Analysis of BKV DNA samples from different time intervals after transplantation demonstrated no significant difference between groups within the first 4 years ().
Figure 4. Serum BKV DNA levels at different time intervals during the early post-transplantation period in asymptomatic renal allograft patients.
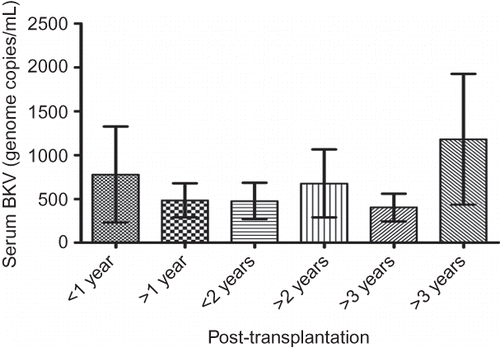
Figure 5. Graft renal biopsy of a patient with typical PVAN. (A) The presence of intranuclear basophilic viral inclusion body in tubular epithelial cell. H&E ×400. (B) Immunohistochemical staining was positive for large T antigen of BKV. ×200.
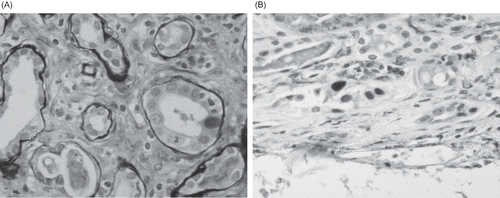
On the other hand, BKV DNA levels of patients with biopsy-proven PVAN were at least 2 logs higher (data not shown). shows the typical biopsy feature of a 52-year-old patient with allograft dysfunction due to PVAN. Her blood BKV DNA load was 1.37 × 106 copies/mL at the time of diagnosis, and immunohistochemical staining was positive for large T antigen of BKV.
DISCUSSION
In our prospective study, the mean prevailing level of serum BKV DNA in renal transplant recipients with good and stable allograft function in the early post-transplantation period was quantifiably low. This is in concert with previous studies, which identified a higher quantifiable BK viral load of ≥1 × 104 genome copies/mL to suggest PVAN.Citation7 However, we have noted a wide interindividual variation in viremia in this cohort. This may be explained by interindividual variation in either a difference in the susceptibility to viral activation or alternatively, a difference in the virulence associated with different BKV strains.Citation14 Furthermore, what is unknown is the serological status and antibody titers of the transplant donors and recipients in this study, and whether the delineation of a primary as opposed to a secondary infection may account for the variability in viremia.Citation15,16
The findings of this study may have useful clinical implications on many levels. At present, there is no prophylactic therapy validated for BKV-associated nephropathy. Therefore, our identification of the absolute background level of BKV DNA in asymptomatic renal allograft recipients provides a benchmark level from which successful screening strategies, based on viral load, can be developed and validated. Such a clinical tool would allow timely adjustment of immunosuppression, which may prevent progression of viremia to PVAN and preclude the need for invasive procedures.Citation17 Although no comparative trials have been performed to date, current evidence does suggest a surveillance approach with the use of preemptive treatment is superior to therapy for established PVAN.Citation18 Moreover, it has been shown that an early diagnosis before the onset of clinically apparent nephropathy is associated with improved outcomes in this entity, which portends a poor prognosis.Citation2,19
The current practice in the management of PVAN involves the judicious manipulation of immunosuppression,Citation9,10 once the diagnosis has been confirmed by tissue biopsy.Citation2,7 However, insufficient decrement of immunosuppression prevents adequate disease control, whereas over reduction is associated with an increased risk of allograft rejection.Citation5 Since BKV DNA levels serve as a quantifiable surrogate marker of disease course,Citation11 our study results allow the guidance of optimal immunosuppressive modulation in PVAN cases.
In addition, the best method of immunosuppressive modulation to achieve disease control remains ill-defined. Different centers have reported different approaches, and there have been variable results with both immunoreduction and class switching.Citation2,20 Our quantification of BK viremia in renal transplant patients with stable graft function may therefore provide useful baseline data, with which comparative clinical trials can use to delineate whether decrement or class switching of immunosuppression is superior.
In conclusion, the results of this study indicate that background BK viral load is quantifiable via Q-PCR. The absolute level of BKV DNA is not a function of time after transplantation and among asymptomatic renal transplant patients, a majority will have serum BKV DNA levels of ≤1 × 103 copies/mL in contrast to levels often reaching 106 copies/mL in patients with PVAN. These findings have clinical implications in the way clinicians manage BKV-associated nephropathy. Screening for BKV replication on a regular basis after transplantation may be of benefit. Moreover, the modulation of immunosuppression against the prevailing level of BK viral load, in patients who have developed PVAN, may achieve disease control without excessive risk of acute graft rejection. Further studies will need to be conducted with regard to finding the best form of immunosuppressive modulation.
ACKNOWLEDGMENTS
This study was supported by the S.K. Yee Medical Foundation. Part of the data contained in this study was presented in abstract form at the XXII International Congress of The Transplantation Society, Sydney, Australia, 10–14 August 2008. The authors are grateful to Ms. Sandra Luen for coordination of sample collection.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Dall A, Hariharan S. BK virus nephritis after renal transplantation. Clin J Am Soc Nephrol. 2008;3(Suppl. 2):S68–S75.
- Hirsch HH, Brennan DC, Drachenberg CB, . Polyomavirus-associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation. 2005;79: 1277–1286.
- Leung AY, Chan M, Tang SC, Liang R, Kwong YL. Real-time quantitative analysis of polyoma BK viremia and viruria in renal allograft recipients. J Virol Methods. 2002;103:51–56.
- Egli A, Binggeli S, Bodaghi S, . Cytomegalovirus and polyomavirus BK posttransplant. Nephrol Dial Transplant. 2007;(Suppl. 8):viii72–viii82.
- Ramos E, Drachenberg CB, Papadimitriou JC, . Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13:2145–2151.
- Drachenberg CB, Beskow CO, Cangro CB, . Human polyoma virus in renal allograft biopsies: Morphological findings and correlation with urine cytology. Hum Pathol. 1999;30:970–977.
- Drachenberg CB, Papadimitriou JC. Polyomavirus-associated nephropathy: Update in diagnosis. Transpl Infect Dis. 2006;8: 68–75.
- Nickeleit V, Klimkait T, Binet IF, . Testing for polyomavirus type BK DNA in plasma to identify renal allograft recipients with viral nephropathy. N Engl J Med. 2000;342:1309–1315.
- Celik B, Shapiro R, Vats A, Randhawa PS. Polyomavirus allograft nephropathy: Sequential assessment of histologic viral load, tubulitis, and graft function following changes in immunosuppression. Am J Transplant. 2003;3:1378–1382.
- Saad ER, Bresnahan BA, Cohen EP, . Successful treatment of BK viremia using reduction in immunosuppression without antiviral therapy. Transplantation. 2008;85:850–854.
- Hirsch HH, Knowles W, Dickenmann M, . Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496.
- Leung AY, Suen CK, Lie AK, Liang RH, Yuen KY, Kwong YL. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood. 2001;98: 1971–1978.
- Orlando C, Pinzani P, Pazzagli M. Developments in quantitative PCR. Clin Chem Lab Med. 1998;36:255–269.
- Smith RD, Galla JH, Skahan K, . Tubulointerstitial nephritis due to a mutant polyomavirus BK virus strain, BKV(Cin), causing end-stage renal disease. J Clin Microbiol. 1998;36:1660–1665.
- Andrews CA, Shah KV, Daniel RW, Hirsch MS, Rubin RH. A serological investigation of BK virus and JC virus infections in recipients of renal allografts. J Infect Dis. 1988;158:176–181.
- Bohl DL, Storch GA, Ryschkewitsch C, . Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5:2213–2221.
- Randhawa P, Brennan DC. BK virus infection in transplant recipients: An overview and update. Am J Transplant. 2006;6: 2000–2005.
- Brennan DC, Agha I, Bohl DL, . Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–594.
- Ramos E, Hirsch HH. Polyomavirus-associated nephropathy: Updates on a persisting challenge. Transpl Infect Dis. 2006;8: 59–61.
- Wali RK, Drachenberg C, Hirsch HH, . BK virus-associated nephropathy in renal allograft recipients: Rescue therapy by sirolimus-based immunosuppression. Transplantation. 2004; 78:1069–1073.