Abstract
Background: Peritoneal transport status is important not only for prescription, but also as a prognostic index. Flt-1 and Flk-1, the major vascular endothelial growth factor receptors involved in angiogenesis and hyperpermeability, may play a potent role in determining peritoneal transport characteristics. However, the relationship between them has not been studied to date. We hypothesized that Flt-1 and Flk-1 expression in the peritoneal vasculature of uremic patients could be closely related to baseline peritoneal transport status. Methods: Thirty-six new patients without a previous history of peritonitis were enrolled. Clinical parameters such as age, sex, height, weight, causes of renal failure, and residual renal function were assessed. Parietal peritoneal biopsies were obtained during implantation of peritoneal dialytic catheters. Flt-1 and Flk-1 were semi-quantitatively evaluated by immunohistochemical staining. Peritoneal microvascular density (MVD) was counted. Within 6 weeks after commencing peritoneal dialysis, a standard peritoneal equilibration test was performed, and the dialysate-to-plasma concentration ratio for creatinine at 4 h (D4/P Cr) was determined. The patients were divided into two groups based on the D4/P Cr: more than 0.65 (Group H, n = 22) and less than or equal to 0.65 (Group L, n = 14). The 24-h peritoneal protein excretion (PPE) was assayed. Flt-1 and Flk-1 were correlated with peritoneal MVD, D4/P Cr, and PPE. Results: Flt-1 and Flk-1 were detected in the peritoneal vasculature of uremic patients. Flt-1 expression was similar between the two groups, but Flk-1 expression in Group H was significantly higher than that in Group L (p = 0.001). Flt-1 expression did not show significant correlations with peritoneal MVD, D4/P Cr, and PPE. However, Flk-1 expression showed significant correlations with the above three parameters (p < 0.001 for all). Conclusions: For the first time, the expressions of Flt-1 and Flk-1 in peritoneal vasculature of uremic patients were detected. Flk-1 expression in peritoneal vasculature of uremic patients is closely correlated with the number of peritoneal microvessels, peritoneal small solute transport rate, and PPE. Our findings strongly suggest that Flk-1 may be a crucial determinant of baseline peritoneal transport characteristics. Further interventional studies are needed.
INTRODUCTION
Patients starting peritoneal dialysis (PD) vary significantly in peritoneal solute transport. Peritoneal transport property determines a patient’s dialysis prescription, as high transporters often need short dwells, high-glucose-containing dialysis fluids, or icodextrin. In addition, many reports have shown an association between high transport status and poor patient or technique survival.Citation1–3 High peritoneal solute transport rate reflects either a large effective peritoneal surface area, which results from a large number of perfused peritoneal capillaries engaged in the diffusion of the solute, or increased permeability of the peritoneal vasculature.
Vascular endothelial growth factor (VEGF) is a potent cytokine that binds to specific receptors on the endothelial cells lining blood vessels. The signaling cascade triggered eventually leads to the formation of new capillaries, a process called angiogenesis. Distributions of VEGF receptors (VEGFRs) and VEGF ligands are therefore the key determinants of angiogenic events.Citation4 In addition, VEGF dramatically increases endothelial cell permeability and is established as one of the most potent endothelial-permeabilizing agents identified thus far.Citation5
Therefore, we have reason to believe that VEGF and VEGFRs play a critical role in determining peritoneal transport characteristics. Indeed, some studies have confirmed that VEGF are associated with high peritoneal solute transport rate.Citation6,7 However, VEGFR is yet to be investigated to date.
In this context, we selected VEGFR1/Flt-1 and VEGFR2/Flk-1, the major isoforms expressed in the endothelial cells, and correlated them with baseline peritoneal transport status as determined by peritoneal equilibration test (PET) and 24-h peritoneal protein excretion (PPE) soon after the commencement of PD.
PATIENTS AND METHODS
Patients
This was a single-center study. During the study period (October 2005 to March 2007), 47 new patients entered the PD program in the Peritoneal Dialytic Unit of Capital Medical University Affiliated Beijing Friendship Hospital. Among them, 36 patients who gave the written consent were included in the study unless they met the exclusion criteria. Age, sex, prevalence of diabetes, and PET profiles of the 36 patients were shown to be representative of the total patient population (data not shown). They were all on continuous ambulatory peritoneal dialysis and received 4 × 2 L exchanges per day; 1.36% dianeal dialysate was used in the daytime, whereas in the long dwell at night 1.36% or 2.27% dianeal dialysate was allowed based on fluid status. All PD solutions were provided by Baxter Healthcare (Guangzhou) Co. Ltd., Guangzhou, China. Subjects with failed renal allograft or who switched from maintenance hemodialysis were excluded. Those with a history of PD-related peritonitis before or during the PET were also excluded. The institutional review board approved the study protocol and informed consent was obtained from all patients.
Clinical Parameters and Assessment of Peritoneal Solute Transport
Detailed demographic and clinical information and PD-associated parameters were obtained. In all patients, baseline peritoneal transport for small solute was evaluated by a standard PET within 4–6 weeks (4.8 ± 0.9 weeks) after starting PD. In short, after an overnight dwell with 1.36% dianeal dialysate, patients were subjected to a 4-h dwell with 2.27% dianeal dialysate. Dialysate samples were taken at 0, 2, and 4 h during the test. Blood samples were taken at 2 h. The dialysate over plasma concentration ratio for creatinine at 4 h (D4/P Cr) was determined. Serum and dialysate creatinine were measured using a kinetic alkaline picrate (Jaffe) method with a sensitivity of 0.2 mg/dL (18 mmol/L). Dialysate creatinine concentration was corrected for glucose interference. Based on D4/P Cr, patients were divided into two groups: high–high average transport group (abbreviated Group H)—D4/P Cr was more than 0.65; low–low average transport group (abbreviated Group L)—D4/P Cr was less than or equal to 0.65.
Urine and dialysate collections (24 h) were performed for measurement of residual renal function and protein excretion through dialysate.
Peritoneal Biopsy
At the time of implanting catheters, parietal peritoneal membranous specimens were obtained. The size of the specimens was required to be similar, which was 0.3 × 0.3 cmCitation2. The specimen was smoothed cautiously, and then was fixed using 4% paraformaldehyde and imbedded with paraffin. For each specimen, using immunohistochemical technique microvascular density (MVD) was counted and the expressions of Flt-1 and Fkt-1 were semi-quantitatively measured.
Immunohistochemistry
Immunohistochemical Strept Avidin-Biotin Complex (SABC)method was used. Positive controls were described above. Phosphate buffer solution (PBS) takes the place of the first antibody, which is the negative control. Peritoneal specimens were sectioned at 10 μm in paraffin wax, and then the wax was removed with water. The sections were treated with fresh 3% hydrogen peroxide at room temperature for 10 min, and washed with distilled water 3 times for 5 min each time. Subsequently, the specimens were incubated by 0.1% trypsin solution for 10 min at 37°C to repair antigens, and washed with PBS 2 times for 3 min each time; 5% bovine serum albumin (BSA)-confining liquid was added at room temperature for 20 min without washing. The first antibody was added to a concentration of 1:100, and left at 4°C overnight. On the next day, at first, the specimens were washed with PBS 3 times for 2 min each time. The second antibody was added at 37°C for 20 min and washed 3 times with PBS for 2 min each time. SABC was added at 37°C for 20 min and then washed with adequate PBS. In Diaminobenzidine (DAB) staining, color intensity was controlled by time under microscopic observation, and slides were washed with flowing water to end the reaction. Slides were dehydrated, cleared, and coverslipped.
ZLI-9010 Trypsin Concentrate and Diluent was bought from Beijing Zhongshan Goldenbridge Biotechnology Limited Company (Beijing, China). Rabbit antihuman Flt-1 polyclonal antibody (No. sc-316) was supplied by Santa Cruz Biotechnology Incorporation (Santa Cruz, CA, USA). Rabbit antihuman Flk-1 polyclonal antibody (No. BA0486), rabbit antihuman CD34 polyclonal antibody (as a marker for vascular endothelial cells, No. BA0532), SABC Immunohistochemical Kit (No. SA1022), DAB Substrate Kit (No. AR1022), and the positive comparison sections for Flk-1, Flt-1, and CD34 were from Wuhan Boster Bioengineering Limited Company (Wuhan, China).
Semi-quantitatively Measuring Expressions of Flt-1 and Flk-1
The slides were observed with high-power microscope (×400). Beginning with the first complete countable visual field from the left superior corner of the sections, the sections were transversely observed from left to right, up to the last countable visual field in this line, then transferred to the next line and observed from right to left. The observation was circularly performed like this. The visual field containing positive staining was taken as a “hot spot.” All of the hot spots were shot. The relative optical density (OD) of each hot spot was measured by means of Image-Pro Plus 5.0 (Media Cybernetics, Inc., Bethesda, MD, USA). The relative OD (abbreviated as OD below) means that the OD was measured after setting the background zero in order to eliminate staining interferences. The sum of OD for all of the hot spots on each section was defined as the semi-quantitative value for the expressions of both receptors.
Measuring Peritoneal Microvascular Density
CD34 was the specific marker for the vascular endothelial cells. After immunohistochemical staining of the sections, MVD of each section was counted. As long as the diameter of some vessels was more than that of eight red blood cells or the vessel wall contained smooth muscles, the vessel was excluded.Citation8 Similarly, beginning with the first complete countable visual field from the left superior corner of the sections, the sections were transversely observed from left to right, up to the last countable visual field in this line, then transferred to the next line and observed from right to left. The observation was circularly performed like this. The counted visual field was selected from every two visual fields. The number of microvessels was recorded for each selected visual field. Their mean value was taken as MVD for this section (numbers/high power (HP)).
Statistic Analysis
Normality of distribution for each variable was assessed by the Kolmogorov–Smirnov test. For continuous variables, comparison between two groups was made by using Student’s t-test. Chi square test was used for categorical variables. A p-value <0.05 was considered statistically significant. SPSS version 11.5 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
RESULTS
Clinical Characteristics
Group H included 22 patients (13 males and 9 females), with a mean age of 61.8 ± 9.7 years (range 44–77). Causes of renal failure were as follows: chronic glomerulonephritis (n = 11), chronic interstitial nephritis (n = 3), diabetic nephropathy (n = 4), lupus nephritis (n = 1), and hypertensive nephrosclerosis (n = 3). Group L recruited 14 patients (7 males and 7 females) whose mean age was 56.0 ± 9.3 years (range 45–72). The underlying renal diseases were chronic glomerulonephritis in nine patients, chronic interstitial nephritis in three patients, and diabetic nephropathy in two patients. Comparisons of clinical characteristics among peritoneal transport categories are shown in .
Table 1. Comparison of clinical characteristics between the peritoneal transport groups of 36 peritoneal dialysis patients.
Table 2. Comparison for Flt-1 expression.
Table 3. Comparison for of Flk-1 expression.
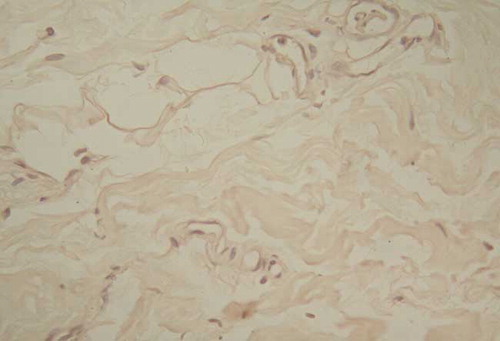
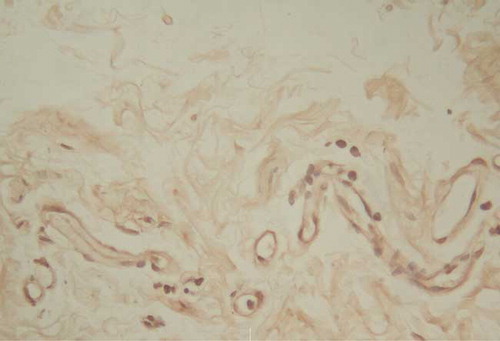
The Immunohistochemical Staining of Peritoneal Flt-1 Location
The positive staining was situated at vascular endothelia, especially microvascular ones; that of arteries and veins was stained very light ( and ).
Comparison between the two groups
As listed in , Flt-1 expression was similar between the two groups (OD: Group H, 0.076 ± 0.024; Group L, 0.070 ± 0.003; p = 0.461).
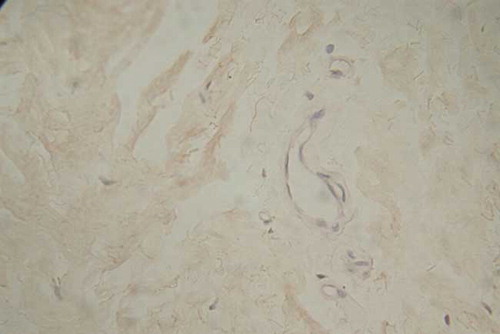
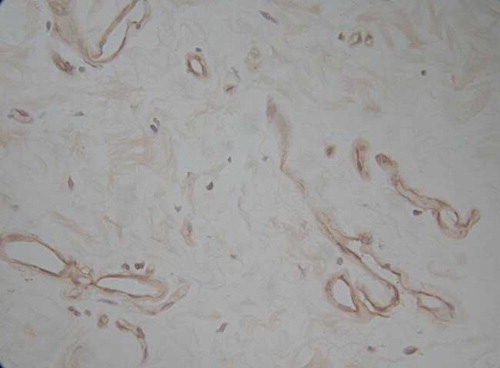
The Immunohistochemical Staining of Peritoneal Flk-1
Location
The positive staining was situated at vascular endothelia; microvascular ones were stained more dark. The specific staining was still observed at the endothelia of some venules; in contrast, the arteries were seldom stained ( and ).
Comparison between the two groups
Flk-1 expression in Group H was significantly higher than that in Group L (OD: Group H, 0.078 ± 0.020; Group L, 0.056 ± 0.012; p = 0.001; see ).
The relationships between VEGFRs and peritoneal MVD
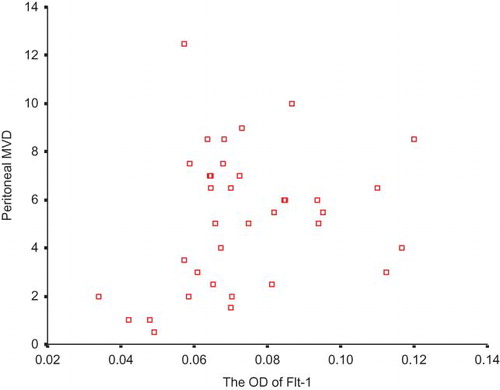
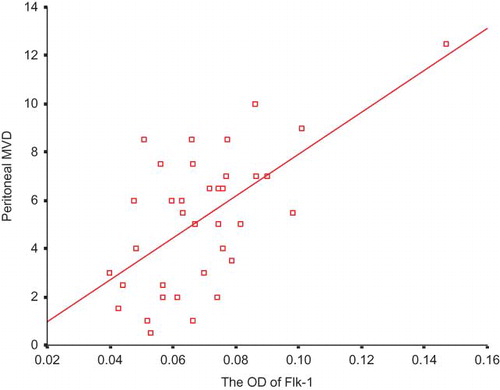
The vascular endothelia were marked by CD34 (). No association between the expression of Flt-1 and peritoneal MVD was discovered (). A positive significance was found between the expression of Flk-1 and peritoneal MVD (ρ = 0.618, p < 0.001; ).
The relationships between Flt-1 and peritoneal transport
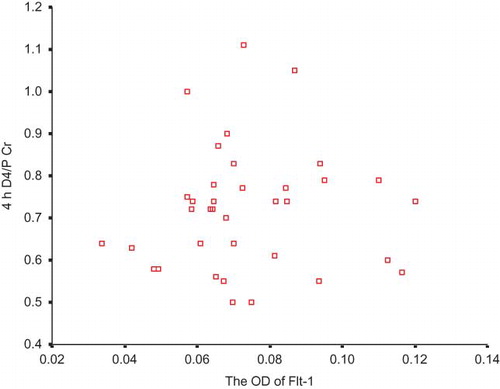
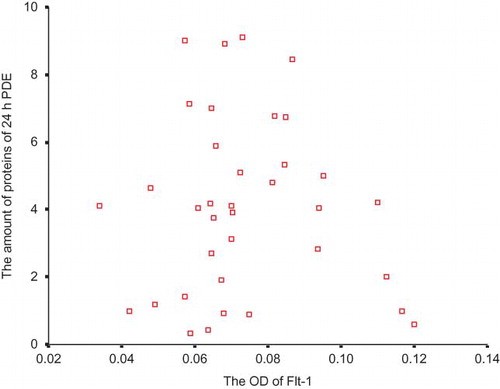
None of the correlations between the expression of Flt-1 and peritoneal transport parameters was significant ( and ).
The relationships between Flk-1 and peritoneal transport
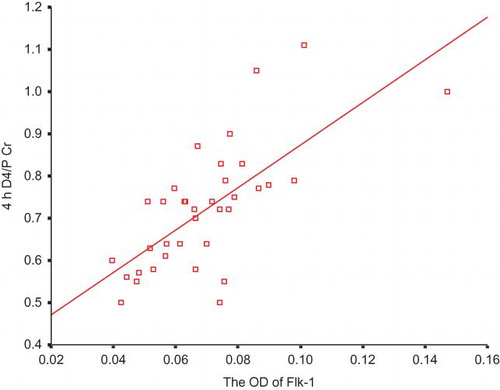
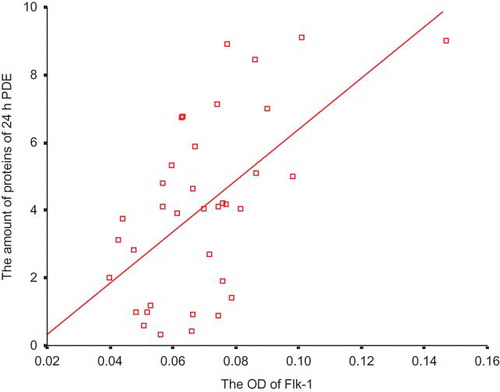
A significant positive correlation between the expression of Flk-1 and the value of D4/P Cr was discovered (ρ = 0.700, p < 0.001; ). There was also a significant positive correlation between the expression of Flk-1 and the amount of proteins in the 24-h peritoneal dialysate effluent (PDE) (ρ = 0.586, p < 0.001; ).
DISCUSSION
For the first time we evaluated the expressions of Flt-1 and Flk-1 in the peritoneal membrane of uremic patients, with the aim of analyzing the relationship between VEGFRs and baseline peritoneal solute transport status. Our results showed that baseline peritoneal transport represented by PET and PPE significantly correlated with Flk-1, but not with Flt-1. Moreover, Flk-1, rather than Flt-1, was positively associated with peritoneal MVD. These results indicate that Flk-1 may be involved in the regulation of peritoneal angiogenesis and microvascular permeability in uremic context. Flk-1 may be a potent determinant of baseline peritoneal transport characteristics.
Peritoneal microvessels are the main barrier to peritoneal solute transport.Citation9 Accordingly, in PD patients with a high baseline peritoneal solute transport rate, a large effective peritoneal surface area, which results from a large number of perfused peritoneal capillaries, or increased permeability of the peritoneal vasculature should be present. VEGF family (VEGF and its receptors) is the central regulator in the development of microvascular hyperpermeability and neoangiogenesis. Therefore, VEGF family might play an influential role in determining baseline peritoneal transport status. The contribution of VEGF ligands has been well described by some investigators.Citation6,7,10 However, till now, there was lack of any evidence relating to VEGF receptors.
The receptors identified so far are designated as VEGFR-1 (fms-like tyrosine kinase, Flt-1), VEGFR-2 (fetal liver kinase-1, Flk-1), VEGFR-3 (fms-like tyrosine kinase 4, Flt4), and the neuropilins (NP-1 and NP-2).Citation11 In adults, Flt-1 and Flk-1 are the major VEGFRs expressed in the blood vascular endothelium. So, we selected Flt-1 and Flk-1 for analysis.
For the first time, we detected the expression of Flt-1 and Flk-1 in peritoneal vasculature of uremic patients. Specific staining could be found in all kinds of vascular endothelia, including arteries, veins, and microvessels. This corresponds with the observed signs in other fields such as oncology, ophthalmology, cardioangiology, and gynecology.Citation12–16 The darkest staining was found in the endothelial cells of microvessels. This consists of the pro-angiogenic activity of VEGFRs, because the formation of new capillaries always begins with the degradation of microvascular basement membranes, followed by proliferation and migration of endothelial cells.
Based on semi-quantitative analyzing, Flt-1 expression had no significant difference between Group H and Group L. In contrast, the expression of Flk-1 in Group H was significantly higher than that of Group L. Further correlating analysis accorded with this result. Flt-1 expression showed no significant association with both the peritoneal MVD and small-molecular solute transport rate (represented by D4/P Cr), but that of Flk-1 showed significant positive correlation with these two parameters.
Actually, it is controversial how Flt-1 influences the endothelial biologic behavior. Fong et al.Citation17 reported that Flt-1-null mutant mice die at embryonic stage E-8.5-9.0 due to the overgrowth and disorganization of blood vessels and not due to a poor vascularization. This result strongly suggests that Flt-1 plays a negative role by suppressing pro-angiogenic signals in the early embryo to establish a balance essential for physiological angiogenesis. Zeng et al.Citation18,19 again demonstrated Flt-1 had the inhibitory effect on the endothelial proliferation, and even illuminated its signaling pathway. In our study, this kind of tendency was not observed. Other animal experiments suggested that Flt-1 was able to facilitate the neovascularity and the establishment of collateral circulations in the ischemic tissues.Citation20 Nevertheless, this effect is mainly mediated by placenta growth factor instead of VEGF.Citation21 The recent view believes that Flt-1 plays a dual role: a negative role in angiogenesis in the embryo and a positive role in adulthood, and the latter acts as a stimulator for carcinogenesis, inflammation, and atherosclerosis.Citation22 Chronic inflammation and atherosclerosis are both highly prevalent in uremic patients. So, it is possible that the negative results from the present study are a comprehensive effect produced by the pros and cons. Certainly, the opinion needs to be confirmed by further studies. In addition, some researchers state that the Flt-1 signaling pathway may regulate normal endothelial cell–cell or cell–matrix interactions and take part in the endothelial migration during vascular development.Citation23 To summarize, there is diversity in the biologic activity of Flt-1. The influence of Flt-1 on the numbers of peritoneal microvessels and peritoneal small solute transport rate in PD patients needs to be elucidated by deeper research in future.
The biologic effect of Flk-1 was well described by Nature in 1995, too.Citation24 Shalaby et al. established the generation of mice deficient in Flk-1 by disrupting the gene using homologous recombination in embryonic stem cells. Embryos homozygous for this mutation died in utero between 8.5 and 9.5 days post-coitum, as a result of an early defect in the development of endothelial cells. Organized blood vessels could not be observed in the embryo or yolk sac at any stage. Their results indicated that Flk-1 was essential for angiogenesis. Now, it is generally acknowledged that Flk-1 is the major mediator of the mitogenic and angiogenic effects of VEGF.Citation25–27 In addition, this receptor has also been proven to be required for the anti-apoptotic effects of VEGF.Citation28–30 After inhibition of this signaling, some normal capillaries regress. Our study showed that the higher the Flk-1 expression in the peritoneal vasculature, the larger its MVD and low-molecular solute transport rate. The results are coincident with the above-mentioned presentations, supporting the potent role of Flk-1 in the interindividual diversity of the baseline peritoneal solute transport status.
VEGF is also named vascular permeability factor (VPF), and it can lead to the increase in the vascular permeability. It is controversial about the receptor type mediating this effect. Murohara et al.Citation31 demonstrated that the interaction of VEGF/VPF with its Flk-1 receptor could give rise to the synthesis of NO and prostacyclin and that the latter two cooperated to enhance vascular permeability. Subsequently, Gille et al.Citation32 made another investigation. They found VEGF mutants that bind selectively to Flk-1 were fully active endothelial cell permeability-enhancing agents, whereas mutants specific for Flt-1 lacked this activity. Now, it is generally believed that Flk-1 but not Flt-1 is the receptor regulating vascular permeability.Citation33 PPE is usually considered to be more closely related with intrinsic permeability of peritoneal vasculature, rather than the effective peritoneal surface area.Citation9 Our results implicated that there was no significant correlation between Flt-1 expression and PPE, whereas Flk-1 expression was significantly associated with PPE. Accordingly, Flk-1 may also be an influential mediator in the peritoneal hyperpermeability of PD patients. The higher the Flk-1 expression is, the higher the peritoneal permeability is, and the more the PPE is, too.
In conclusion, Flk-1 expression in peritoneal vasculature of uremic patients is closely correlated with the number of peritoneal microvessels, peritoneal small solute transport rate, and PPE. Our findings strongly suggest that Flk-1 may be a crucial determinant of baseline peritoneal transport characteristics. Further interventional studies are needed.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Churchill DN, Thorpe KE, Nolph KD, . Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1998;9:1285–1292.
- Wang T, Heimburger O, Waniewski J, Bergström J, Lindholm B. Increased peritoneal permeability is associated with decreased fluid and small-solute removal and higher mortality in CAPD patients. Nephrol Dial Transplant. 1998;13(5):1242–1249.
- Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol. 2006;17(1):271–278.
- Stefanini MO, Wu FT, Mac Gabhann F, . The presence of VEGF receptors on the luminal surface of endothelial cells affects VEGF distribution and VEGF signaling. PLoS Comput Biol. 2009;5(12):e1000622.
- De Vriese AS, Tilton RG, Stephan CC, . Vascular endothelial growth factor is essential for hyperglycemia-induced structural and functional alterations of the peritoneal membrane. J Am Soc Nephrol. 2001;12(8):1734–1741.
- van Esch S, Zweers MM, Jansen MA, . Determinants of peritoneal solute transport rates in newly started nondiabetic peritoneal dialysis patients. Perit Dial Int. 2004;24(6):554–561.
- Rodrigues AS, Martins M, Korevaar IC, . Evaluation of peritoneal transport and membrane status in peritoneal dialysis: Focus on incident fast transporters. Am J Nephrol. 2007;27(1):84–91.
- Neufeld G, Cohen T, Gengrinovitch S, . Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22.
- Numata M, Nakayama M, Nimura S, Kawakami M, Lindholm B, Kawaguchi Y. Association between an increased surface area of peritoneal microvessels and a high peritoneal solute transport rate. Perit Dial Int. 2003;23(2):116–122.
- Yang WS, Tsai TJ, Shih CL, . Intraperitoneal vascular endothelial growth factor C level is related to peritoneal dialysis ultrafiltration. Blood Purif. 2009;28(1):69–74.
- Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: Review. Blood Cells Mol Dis. 2007;38(3):258–268.
- Inoue M, Itoh H, Ueda M, . Vascular endothelial growth factor (VEGF) expression in human coronary atherosclerotic lesions: Possible pathophysiological significance of VEGF in progression of atherosclerosis. Circulation. 1998;98(20):2108–2116.
- Brogi E, Schatteman G, Wu T, . Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. J Clin Invest. 1996;97(2):469–476.
- Witmer AN, Blamwgeers HG, Weich HA. Alter expression patterns of VEGF-receptors in human diabetic retina and in experimental VEGF-induced retinopathy in monkey. Invest Ophthalmol Vis Sci. 2002;43:849–857.
- Mints M, Blomgren B, Falconer C, . Expression of the vascular endothelial growth factor (VEGF) family in human endometrial blood vessels. Scand J Clin Lab Invest. 2002;62(3):167–175.
- Suzuki H, Seto K, Shinoda Y, . Paracrine upregulation of VEGF receptor mRNA in endothelial cells by hypoxia-exposed hep G2 cells. Am J Physiol. 1999;276(1):G92–G97.
- Fong GH, Rossant J, Gertsenstein M, . Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376(6535):66–70.
- Zeng H, Dvorak HF, Mukhopadhyay D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) receptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2001;276(29):26969–26979.
- Zeng H, Zhao D, Mukhopadhyay D. Flt-1-mediated down-regulation of endothelial cell proliferation through pertussis toxin-sensitive G proteins, beta gamma subunits, small GTPase CDC42, and partly by Rac-1. J Biol Chem. 2002;277(6):4003–4009.
- Eriksson U, Alitalo K. VEGF receptor 1 stimulates stem-cell recruitment and new hope for angiogenesis therapies. Nat Med. 2002;8(8):775–777.
- Sawano A, Takahashi T, Yamaguchi S, . Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996;7(2):213–221.
- Kanno S, Oda N, Abe M, . Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000;19(17):2138–2146.
- Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39(5):469–478.
- Shalaby F, Rossant J, Yamaguchi TP, . Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–66.
- Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25(4):581–611.
- Ewan LC, Jopling HM, Jia H, . Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic. 2006;7(9):1270–1282.
- Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114(Pt 5):853–865.
- Baffert F, Thurston G, Rochon-Duck M, . Age-related changes in vascular endothelial growth factor dependency and angiopoietin-1-induced plasticity of adult blood vessels. Circ Res. 2004;94(7):984–992.
- Kamba T, Tam BY, Hashizume H, . VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(2):H560–H576.
- Baffert F, Le T, Sennino B, . Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290(2): H547–H559.
- Murohara T, Horowitz JR, Silver M, . Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97(1):99–107.
- Gille H, Kowalski J, Li B, . Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276(5): 3222–3230.
- Bhattacharya R, Kang-Decker N, Hughes DA, . Regulatory role of dynamin-2 in VEGFR-2/KDR-mediated endothelial signaling. FASEB J. 2005;19(12):1692–1694.