Abstract
Peritoneal fibrosis is a serious complication in patients with severe chronic kidney disease who are undergoing peritoneal dialysis (PD). One of the pathological characteristics of peritoneal fibrosis is the infiltration of macrophages in the thickened submesothelial compact zone. In addition, infiltration of lymphocytes, including T and B lymphocytes, is observed in the fibrotic peritoneum. However, the relationship between lymphocyte infiltration and progression of peritoneal fibrosis remains unclear. In this study, we investigated the role of lymphocytes in the development of peritoneal fibrosis induced by chlorhexidine gluconate (CG) by comparing the histological changes observed in severe combined immunodeficient (SCID) mice (largely lacking functional T and B lymphocytes) with those observed in wild-type (WT) mice. As expected, CG-injected WT mice showed a thickening of the submesothelial compact zone together with massive collagen deposition accompanied by increased numbers of infiltrating macrophages and T and B lymphocytes. In the peritoneum of SCID mice, the submesothelial compact zone was thicker and the number of macrophages and B lymphocytes was significantly higher than that observed in control immunodeficient and WT mice. In contrast, the number of T lymphocytes in the peritoneum of SCID mice was significantly lower than that in the peritoneum of WT mice. These results suggest that T and B lymphocytes modulate the process of peritoneal fibrosis via macrophage infiltration.
INTRODUCTION
Although peritoneal dialysis (PD) is a beneficial therapy for end-stage renal disease, long-term PD causes histopathological changes in the peritoneum, such as peritoneal fibrosis characterized by increased submesothelial collagen deposition and loss of mesothelial cells. Furthermore, recent studies have identified vascularization with vasculopathyCitation1 and macrophage infiltrationCitation2 in the peritoneum of patients undergoing long-term PD therapy. These changes gradually lead to the loss of ultrafiltration and the cessation of PD.Citation3 Therefore, to maintain peritoneal function, it is important to elucidate the mechanism(s) of peritoneal fibrosis. Several animal models have been used to investigate peritoneal fibrosis. Agents used to induce peritoneal fibrosis include lipopolysaccharide,Citation4 acidic PD solution with a pH of 3.8,Citation5 and chlorhexidine gluconate (CG).Citation6,7 The latter two agents caused peritoneal adhesions that were very similar to those seen in patients with encapsulating peritoneal sclerosis (EPS), a serious complication of long-term PD therapy. Although the mechanism underlying the development of peritoneal fibrosis by CG is unclear, previous reports have shown that persistent chemical irritation by CG could induce tissue damage with inflammation characterized by infiltration of macrophages followed by excessive proliferation of fibroblasts.Citation8 As repeated intraperitoneal exposure of experimental rodents to CG is a potentially valuable experimental model for peritoneal fibrosis,Citation9,10 we used CG to induce peritoneal fibrosis in this study.
It has been reported that infiltration of lymphocytes, including T and B lymphocytes, was observed in the fibrotic peritoneum of patients undergoing PD.Citation11 Recently, Wang et al.Citation12 investigated the serial changes in T-lymphocyte subpopulations and cytokine mRNA expression patterns in PD effluents during peritonitis in patients undergoing PD and found that the CD4/CD8 ratio might be related to the outcome of peritonitis in these patients. Moreover, another group reported that a progressive decrease in the CD4/CD8 ratio of PD effluents correlated with a persistent expression of tumor growth factor beta-1 (TGF-β1), which is known to play crucial roles in the development of peritoneal fibrosis.Citation13 These data suggest that T lymphocytes may be involved in the development of peritonitis and peritoneal fibrosis in patients undergoing PD, although the association of B lymphocytes with the peritoneal alteration remains unclear. Severe combined immunodeficient (SCID) mice have a mutation resulting in a lack of functional T and B lymphocytes in both lymphoid organs and the peripheral circulation, while the macrophages are unaffected. For this reason, SCID mice are quite useful for investigating the role of lymphocytes in the pathogenesis of various diseases.Citation14,15 In this study, we investigated the involvement of lymphocytes in the development of peritoneal fibrosis induced by the CG in the SCID mice.
MATERIALS AND METHODS
Animals
Male CB-17/LcrCriBR mice [wild-type (WT)] and CB-17/LcrCri-scid (SCID) mice (Charles River Laboratories Japan, Yokohama, Japan) weighing 25–30 g were used in this study. Animals were housed in standard rodent cages at the Biomedical Research Center, Center for Frontier Life Sciences, Nagasaki University, at a constant ambient temperature (22 ± 1°C) and humidity (85%) under 10 h of light each day. Animals had free access to drinking water and pelleted rodent food. The experimental protocol was approved by the Animal Care and Use Committee of Nagasaki University and was approved by the President of Nagasaki University (approval number: 1003301048).
Experimental Protocol
Peritoneal fibrosis was induced as described previously by Yoshio et al.Citation10 The mice were assigned to four groups: WT-control group (n = 10), SCID-control group (n = 10), WT-CG group (n = 10), and SCID-CG group (n = 10). The mice in the WT-CG group and the SCID-CG group received an intraperitoneal injection of 0.1% CG in 15% ethanol dissolved in 0.2 mL of saline every other day. The mice in the WT-control group and the SCID-control group received an intraperitoneal injection of 15% ethanol dissolved in 0.2 mL of saline every other day. To avoid artifacts from the direct effect of repeated injections, the mice were injected in the lower part of the peritoneum, while the upper portion of the parietal peritoneum was used for histological examination. Mice were killed at day 8 (n = 5, each group) or day 16 (n = 5, each group) after the first CG injection and peritoneal tissues were carefully dissected. The harvested tissues were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) immediately after sampling and embedded in paraffin in a standard manner.
Histological and Immunohistochemical Examination
For morphological examination, 4-μm-thick paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E). These paraffin-embedded tissue sections (4 μm thick) were also stained immunohistochemically with the following antibodies: (1) goat anti-mouse CD3 antibody diluted 1/200 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), used as a marker for pan T cells; (2) rat anti-mouse CD45R antibody diluted 1/100 (Ly-5, B220; clone RA3-6B2, Caltag Laboratories, Bangkok, Thailand), used as a marker for pan B cells; (3) rat anti-mouse F4/80 antibody diluted 1/50 (clone Cl:A3-1, MCA497, Serotec, Oxford, UK), used as a marker of macrophages; and (4) rabbit anti-TGF-β antibody diluted 1/200 (Santa Cruz Biotechnology).
Indirect immunohistochemistry of paraffin sections was performed for CD45R and F4/80 localization. Briefly, deparaffinized tissue sections were incubated for 30 min with a blocking buffer containing 20% normal swine serum, 5% normal goat serum, 5% fetal calf serum, and 5% bovine serum albumin in phosphate-buffered saline. The sections were then exposed to primary antibody, diluted in the same blocking buffer. After reacting with anti-CD45R antibody or anti-F4/80 antibody for 1 h at room temperature, the sections were exposed to horseradish peroxidase-conjugated rabbit anti-rat antibody (P450, Dako, Glostrup, Denmark) and horseradish peroxidase-conjugated swine anti-rabbit antibody (P399, Dako).
For CD3 and TGF-β localization, deparaffinized tissues were stained with avidin–biotin complex using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) after reacting with primary antibody for 1 h at room temperature. Reaction products were visualized by treating sections with hydrogen peroxide and 3,3′ diaminobenzidine tetrahydrochloride. Finally, the sections were counterstained with methyl green and mounted. As negative controls, sections were incubated with any irrelevant mouse or rat monoclonal antibody, or normal rabbit IgG in place of the primary antibody.
Data Processing and Statistical Analysis
To assess the extent of peritoneal thickening, we used digitized images and image analysis software (WinROOF, MITANICORP, Chiba, Japan). We measured the thickness of the submesothelial zone above the abdominal muscle in cross-sections of the abdominal wall. The image was transformed into a matrix of 1280 × 1000 pixels and viewed at 100× magnification. We selected a width of 840 μm in the examined field and measured the area of the submesothelial layer within the selected area. For each sample, eight such areas were selected and the average thickness of the submesothelial layer was determined. Fibrotic areas in the peritoneum were quantified on sections stained with Azan-Mallory dyes to visualize the collagen fibers (stained in blue). Under 100× magnification, eight consecutive non-overlapping fields were analyzed. Blue fibrotic areas were detected on digital images using a computer-assisted image analyzer, and the fibrotic area was digitized. In each peritoneal sample, the number of cells positive for CD3, CD45R, F4/80, and TGF-β was counted in 10 fields at 400× magnification. Data were expressed as mean ± SD. Differences among groups were examined for statistical significance using repeated-measures ANOVA (Bonferroni/Dunn test). p-Value less than 0.05 was considered statistically significant.
RESULTS
Morphological Examination
Morphological changes were assessed by H&E staining. In WT mice, the peritoneal tissue consisted of the peritoneal mesothelial monolayer with an exiguity of connective tissues under the mesothelial layer (A). There were no appreciable differences in the peritoneal tissues between WT and SCID mice before CG injections (data not shown). In the control groups (WT-control and SCID-control groups), the peritoneal tissues were almost normal without any thickening of the submesothelial compact zone (data not shown).
Figure 1. Hematoxylin and eosin staining of peritoneal tissues. The monolayer of mesothelial cells that covers the surface of peritoneum in normal mice (A). Chlorhexidine gluconate (CG) injection induced significant thickening of the peritoneum at day 8 (B) and day 16 (C) in WT mice. In SCID mice, the peritoneal tissues became thicker than that of WT mice at day 8 (D) and day 16 (E). Bars indicate the thickness of the submesothelial compact zone. Magnification, ×100. The average thickness of the submesothelial compact zone in WT control mice (WT-control), CG-injected WT mice (WT-CG), SCID-control mice (SCID-control), and CG-injected SCID mice (SCID-CG) at day 16 (F). Data are presented as the mean ± SD. a: p < 0.01 versus WT-control; b: p < 0.01 versus SCID-control; c: p < 0.01 versus WT-CG.
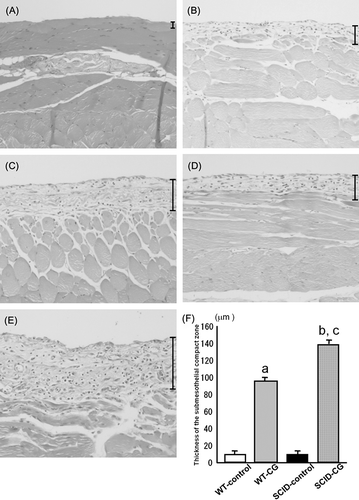
On the other hand, the peritoneal tissues in WT-CG group showed a marked thickening of the submesothelial compact zone and the presence of numerous infiltrating cells at day 8 and day 16 (B and C). Moreover, the thickness of the submesothelial compact zone in the SCID-CG group was significantly increased compared to that observed in the control and WT-CG groups at day 8 and day 16 (D–F).
Infiltration of Macrophages, T Lymphocytes, and B Lymphocytes
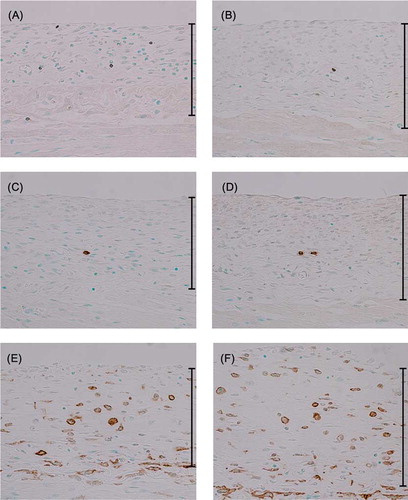
To identify the type of inflammatory cells infiltrating the submesothelial compact zone, immunohistochemistry was performed on peritoneal specimens with anti-CD3 antibody as a marker for T lymphocytes, anti-CD45R antibody as a marker for B lymphocytes, and anti-F4/80 antibody as a marker for mouse macrophages. In the WT-CG group, the number of CD3-positive T lymphocytes (A) and CD45R-positive B lymphocytes (C) was increased and a large number of F4/80-positive macrophages (E) were observed in the thickened peritoneal tissues compared to the WT-control group at day 16 (). The peritoneal tissue in the SCID-CG group showed a significantly increased number of B lymphocytes and macrophages compared to the WT-CG group at day 16 (D and F). In contrast, the number of T lymphocytes in the peritoneum in the SCID-CG group was significantly lower than that in the WT-CG group (B).
Table 1. The number of T lymphocytes, B lymphocytes, or macrophages in the submesothelial layer.
Figure 2. Immunohistochemistry for CD3, CD45R, and F4/80 at day 16. Immunohistochemical detection of CD3-positive (A), CD45R-positive (C) and F4/80-positive (E) cells in the peritoneum of CG-injected WT mice. CG-injected SCID mice were also analyzed for the presence of CD3-positive (B), CD45R-positive (D), and F4/80-positive (F) cells. Magnification, ×200.
Expression of TGF-β
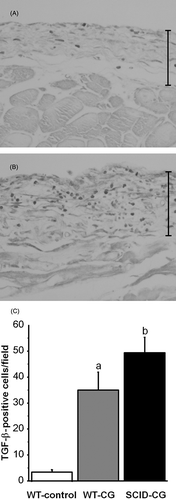
We examined the expression of TGF-β—a cytokine which plays an important role in tissue fibrosis—by immunohistochemistry.Citation13 The number of TGF-β-positive cells was significantly higher in the thickened peritoneum in the WT-CG group than in the control groups (A and C). It should be noted that the number of TGF-β-positive cells in the SCID-CG group was significantly higher than that of the control and WT-CG groups (B and C).
Figure 3. Immunohistochemistry for TGF-β. In CG-injected WT mice, TGF-β-positive cells are present in thickened submesothelial compact zone (A). In CG-injected SCID mice, the number of TGF-β-positive cells is increased compared to CG-injected WT mice (B). Magnification, ×200. The number of TGF-β-positive cells was determined in 10 fields (magnification, ×400) selected at random in the submesothelial region (C). Data represent the mean ± SD. a: p < 0.01 versus control-treated WT mice (WT-control); b: p < 0.01 versus WT-control and CG-injected WT mice (WT-CG).
DISCUSSION
In this study, we have demonstrated that, in addition to macrophages, T and B lymphocytes also infiltrated the thickened submesothelial compact zone in the peritoneal fibrosis mouse model induced by CG. Furthermore, when we induced peritoneal fibrosis in SCID mice, the mice developed far more severe fibrosis compared to CG-injected WT mice, with marked macrophage infiltration in the submesothelial compact zone. Moreover, the number of TGF-β-positive cells in the SCID-CG group was significantly increased compared to that of the control and WT-CG groups. Therefore, these results suggest that the CG-induced peritoneal fibrosis in a mouse model may accurately reflect features observed in the fibrotic peritoneum of patients undergoing long-term PD therapy and that T and B lymphocytes may modulate the process of peritoneal fibrosis via macrophage infiltration and TGF-β expression.
Macrophages, neutrophils, T lymphocytes, and B lymphocytes have been observed in the fibrotic peritoneum of humans.Citation11 In addition, it was reported that CD4-positive and CD8-positive T lymphocytes were present in the peritoneum of patients with EPS, whereas only sparse numbers of B lymphocytes were found in the fibrotic peritoneum without EPS.Citation11 Despite these important observations, the involvement of infiltrating lymphocytes in the progression of peritoneal fibrosis had not been fully elucidated.
Recently, the involvement of infiltrating T lymphocytes in various diseases, such as lung fibrosis,Citation16 muscle degeneration,Citation14 and renal interstitial fibrosis,Citation17 has been reported. Chung et al.Citation18 reported the possible involvement of T cells in the pathogenesis of peritoneal adhesions, which are commonly observed in EPS. Using a surgical and a post-infection adhesion model of the peritoneum, the experiments with a deletion and replenishment of CD4-positive T lymphocytes revealed that adhesion formation is dependent on CD4-positive αβ T lymphocytes.Citation18 Moreover, the investigators reported that the immunopathogenesis of adhesion formation is under the control of T lymphocytes and that T lymphocyte-derived cytokines and chemokines play important roles in the development of this deleterious host response.Citation18
In contrast, the connection between B lymphocytes and fibrosis has been controversial. In a bleomycin-induced pulmonary fibrosis mouse model, the development of fibrosis was associated with CD19-dependent signals controlling B-lymphocyte infiltration into the lung tissue.Citation19 In the kidney, although the relative percentage of B lymphocytes in the interstitium of chronic diseased kidney was lower than that of macrophages and T lymphocytes, a significant negative correlation between B lymphocytes and the degree of renal function was observed.Citation20,21
As SCID mice have virtually no mature T or B lymphocytes in both lymphoid organs and the peripheral circulation, the functions attributable to T- or B-lymphocyte action are absent in these mice.Citation22 Recently, Shappell et al.Citation15 investigated the role of lymphocyte infiltration in the development of tubulointerstitial injury induced by unilateral ureter ligation in SCID and WT mice. In their study, WT and SCID mice developed tubular atrophy and interstitial volume expansion in the ligated kidney to the same degree and at the same rate. These results indicated that lymphocytes were not necessary for the development of progressive tubular injury and interstitial fibrosis in chronic obstructive uropathy.
Using SCID mice, we examined the role of lymphocytes during the development of peritoneal fibrosis induced by CG. Importantly, while SCID mice lack mature B and T cells, their macrophage levels are normal. In this study, we showed that the peritoneal tissue in the SCID-CG group was thicker than that of the control and WT-CG groups. Notably, the number of macrophages in the peritoneum of SCID-CG mice was significantly increased compared to that of the control and WT-CG groups. These results strongly suggest that the principal immune cell component in the development of fibrosis is macrophages, not T lymphocytes or B lymphocytes.
Macrophages play an important role in tissue homeostasis and remodeling in addition to their role as potent immune regulators. In addition, activated macrophages and myofibroblasts have been established as critical cells for the production of TGF-β in peritoneal fibrosis.Citation13 The TGF-β is a multifunctional cytokine involved in many aspects of the wound healing process; fibroblast activation, collagen deposition, inhibition of fibrinolysis through plasminogen activator inhibitor-1, collagenolysis through tissue inhibitor of metalloproteinase-1, and angiogenesis.Citation13 In this study, the number of macrophages and TGF-β-positive cells in the SCID-CG group significantly increased, together with progression of peritoneal fibrosis, compared to that in the control and WT-CG groups. These data further confirm that macrophage infiltration and TGF-β production may contribute to the development of peritoneal fibrosis in SCID mice.
It is well known that the interplay among T lymphocytes, B lymphocytes, and macrophages occurs during the immune response. T-lymphocyte responses can initiate hypersensitivity immune reactions and stimulate macrophages to generate proinflammatory mediators of injury.Citation23 Furthermore, B lymphocytes are also reported to have important roles in antigen presentation and the modulation of regulatory T-lymphocyte numbers and functions in certain settings. These include anti-neutrophil cytoplasmic antibody-positive vasculitisCitation24 and membranous nephropathy,Citation25,26 although it is unclear whether B lymphocytes are directly involved in the macrophage activation. However, these reports do suggest that T and B lymphocytes regulate macrophage infiltration directly and indirectly. Although our results also suggest that T lymphocytes, B lymphocytes or both play an important role in regulating TGF-β production and macrophage infiltration, thereby modulating the development of the peritoneal fibrosis, the precise mechanism(s) that regulate the development of peritoneal fibrosis remain unclear. Future experiments in the absence of either B lymphocytes or T lymphocytes alone should provide valuable insights into the complex immune interactions that control peritoneal fibrosis.
Declaration of interest:The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Williams JD, Craig KJ, Topley N, . Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–479.
- Shioshita K, Miyazaki M, Ozono Y, . Expression of heat shock proteins 47 and 70 in the peritoneum of patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2000;57:619–631.
- Honda K, Nitta K, Horita S, . Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron. 1996;72:171–176.
- Margetts PJ, Kolb M, Yu L, . A chronic inflammatory infusion model of peritoneal dialysis in rats. Perit Dial Int. 2001;21(Suppl. 3):S368–S372.
- Nakamoto H, Imai H, Ishida Y, . New animal models for encapsulating peritoneal sclerosis—role of acidic solution. Perit Dial Int. 2001;21(Suppl. 3):S349–S353.
- Suga H, Teraoka S, Ota K, . Preventive effect of pirfenidone against experimental sclerosing peritonitis in rats. Exp Toxicol Pathol. 1995;47:287–291.
- Ishii Y, Sawada T, Shimizu A, . An experimental sclerosing encapsulating peritonitis model in mice. Nephrol Dial Transplant. 2001;16:1262–1266.
- Mishima Y, Miyazaki M, Abe K, . Enhanced expression of heat shock protein 47 in rat model of peritoneal fibrosis. Perit Dial Int. 2003;23:14–22.
- Nishino T, Miyazaki M, Abe K, . Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress peritoneal fibrosis in rats. Kidney Int. 2003;64:887–896.
- Yoshio Y, Miyazaki M, Abe K, . TNP-470, an angiogenesis inhibitor, suppresses the progression of peritoneal fibrosis in mouse experimental model. Kidney Int. 2004;66:1677–1685.
- Bertoli SV, Barone MT, Vago L, . Changes in peritoneal membrane after continuous ambulatory peritoneal dialysis—a histopathological study. Adv Perit Dial. 1999;15:28–31.
- Wang HH, Lin CY, Huang TP. Patterns of CD4/CD8 T-cell ratio in dialysis effluents predict the long-term outcome of peritonitis in patients undergoing peritoneal dialysis. Nephrol Dial Transplant. 2003;18:1181–1189.
- Margetts PJ, Oh KH, Kolb M. Transforming growth factor-beta: Importance in long-term peritoneal membrane changes. Perit Dial Int. 2005;25(Suppl. 3):S15–S17.
- Farini A, Meregalli M, Belicchi M, . T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. J Pathol. 2007;213:229–238.
- Shappell SB, Gurpinar T, Lechago J, . Chronic obstructive uropathy in severe combined immunodeficient (SCID) mice: Lymphocyte infiltration is not required for progressive tubulointerstitial injury. J Am Soc Nephrol. 1998;9:1008–1017.
- Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukoc Biol. 2008;83:237–244.
- Strutz F, Neilson EG. The role of lymphocytes in the progression of interstitial disease. Kidney Int. 1994;45(Suppl. 45):S106–S110.
- Chung DR, Chitnis T, Panzo RJ, . CD4+ T cells regulate surgical and postinfectious adhesion formation. J Exp Med. 2002;195:1471–1478.
- Komura K, Yanaba K, Horikawa M, . CD19 regulates the development of bleomycin-induced pulmonary fibrosis in a mouse model. Arthritis Rheum. 2008;58:3574–3584.
- Boucher A, Droz D, Adafer E, Noel LH. Characterization of mononuclear cell subsets in renal cellular interstitial infiltrates. Kidney Int. 1986;29:1043–1049.
- Hooke DH, Gee DC, Atkins RC. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987;31:964–972.
- Bosma MJ, Carroll AM. The SCID mouse mutant: Definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350.
- Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530.
- Savage CO. Pathogenesis of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Clin Exp Immunol. 2011;164(Suppl. 1):23–26.
- Cohen CD, Calvaresi N, Armelloni S, . CD20-positive infiltrates in human membranous glomerulonephritis. J Nephrol. 2005;18:328–333.
- Ruggenenti P, Chiurchiu C, Brusegan V, . Rituximab in idiopathic membranous nephropathy: A one-year prospective study. J Am Soc Nephrol. 2003;14:1851–1857.