Abstract
Studies indicate that Syzygium spp-derived oleanolic acid (OA) enhances renal function of streptozotocin (STZ)-induced diabetic rats as evidenced by its reversal of the previously reported inability of the kidney to excrete Na+ in these animals. We postulated that OA influences Na+ excretion in the proximal tubule, the site where two-thirds of filtered NaCl is reabsorbed through a process mediated by transport proteins. Therefore, the study investigated the effects of OA on proximal tubular Na+ handling in male Sprague–Dawley rats using renal lithium clearance (CLi). Renal CLi has been used widely in animal and clinical studies to assess proximal tubular function. Sub-chronic doses of OA were administered to rats twice every third day for 5 weeks. Rats treated with deionized water served as control animals. Cytotoxicity of OA on kidney and liver cell lines was assessed by the MTT and comet assays. OA increased Na+ excretion of conscious male Sprague–Dawley rats from week 3 to week 5. By the end of the 5-week experimental period, OA treatment significantly reduced (p < 0.05) plasma creatinine concentration of STZ-induced diabetic rats with a concomitant elevation in glomerular filtration rate (GFR). Acute OA infusion was also associated with increases in fractional excretion of sodium (FENa) and lithium (FELi) in anesthetized rats in the absence of significant changes in GFR. The MTT assay studies demonstrated that OA increased the metabolic activity of kidney and liver cell lines. Taken together with previous observations, this study implicates the proximal tubule in OA-evoked increases in urinary Na+ output.
INTRODUCTION
Plants containing the triterpene constituent oleanolic acid (3β-hydroxy-olea-12-en-28-oic acid, OA) have several traditional uses in folk medicines in terms of anti-inflammatory, hepatoprotective, analgesic, cardiotonic, sedative, and tonic effects.Citation1,2 However, increasing evidence suggests that OA possesses hypoglycemic properties and may also have beneficial effects on some processes associated with impaired kidney function in diabetes.Citation3,4 Indeed, OA appears to reverse the previously reported inability of the kidney to excrete Na+ in streptozotocin (STZ)-induced diabetic rats.Citation5 These findings suggest that OA may be influencing transport processes in the proximal tubule, the site for the reabsorption of approximately two-thirds of the Na+ that enters the tubular fluid by glomerular filtration. Accordingly, the study investigated the effects of OA on proximal tubular Na+ handling using renal lithium clearance. We acknowledge that the specificity of lithium as a marker of proximal tubule sodium reabsorption is controversial; nonetheless, lithium can be considered to be a good indicator of proximal tubule function.Citation6–10 Available evidence suggests that some herbal extracts interfere with the concentrating and diluting mechanisms of tubular transport processes in the nephron and/or with other protein components of tubular cell membranes.Citation11,12 Therefore, any link between OA affecting proximal tubular Na+ reabsorption and secretion would require that the triterpene influences tubular transport protein components or tubular epithelial cells’ viability. To examine this possibility, this study also assessed the effects of OA on cell viability on human embryonic kidney (HEK293) and Madin–Darby bovine kidney (MDBK) cell lines. Cell culture systems have been extensively used to evaluate toxicity of drugs in specific organs.Citation13 In vitro cell culture techniques that mimic the in vivo state of cell lines maintain similar levels of marker enzymes of metabolism exhibited by freshly isolated cells.Citation14
MATERIALS AND METHODS
Drugs and Chemicals
All chemicals and drugs used for this study were of analytical grade and were purchased from standard commercial and pharmaceutical suppliers, respectively.
Isolation of OA
OA was isolated from Syzygium aromaticum [(Linnaeus) Merrill & Perry] (Myrtaceae) (cloves) using a previously validated protocol in our laboratory.Citation3,4 Air-dried powdered flower buds of S. aromaticum were exhaustively extracted thrice at 24-h intervals sequentially with 3 L of hexane, dichloromethane, ethyl acetate, and methanol at room temperature. The solvents were removed under reduced pressure to give corresponding residues of hexane-solubles, dichloromethane-solubles, ethyl acetate-solubles (EAS), and methanol-solubles. Recrystallization of EAS which had been previously shown to contain OA ursolic acid/methyl maslinate/methyl corosolateCitation3,15 with ethanol yielded pure OA, whose structure was confirmed by spectroscopic analysis using 1D and 2D, 1H and 13C nuclear magnetic resonance techniques. Preliminary studies indicated that the S. aromaticum-isolated OA and commercial OA were similar and hence the plant-extracted OA was used in the experiments as it costs less.
Animals
Male Sprague–Dawley rats (250–300 g body weight) bred and maintained at Biomedical Research Unit, University of KwaZulu-Natal, were used in this study. The animals had free access to standard rat chow (Meadows, Pietermaritzburg, South Africa) and water, with a 12-h light/12-h dark cycle. The experiments were performed in accordance with the institutional guidelines of the University of KwaZulu-Natal.
Experimental Design
The effects of various doses of OA on renal fluid and electrolyte handling were assessed in conscious male Sprague–Dawley rats, while the effects on proximal tubular Na+ handling using lithium as a marker were evaluated in anesthetized rats. In vitro effects of OA were evaluated on HEK293, MDBK, and liver (HepG2) cell lines.
Renal Fluid and Electrolyte Handling Studies
Separate groups of male Sprague–Dawley rats housed individually in Makrolon polycarbonate metabolic cages (Tecniplast, Labotec, South Africa) were treated with various doses of OA (30, 60, and 120 mg kg−1, p.o.) by means of a bulbed steel tube every third day for 5 weeks at 09.00 and 15.00 (n = 7 in each group). OA was freshly dissolved in dimethyl sulfoxide (DMSO, 2 mL) and normal saline (19 mL) before use in each case. Rats treated with DMSO/saline (3 mL kg−1, p.o.) acted as untreated controls. The rats were given both food and water ad libitum. Urine volume and urinary concentrations of creatinine, urea, Na+, K+, and Cl− were determined every third day for 5 weeks at 09.00. Mean arterial blood pressure (MAP) was monitored every third consecutive day for 5 weeks at 09.00 using non-invasive tail cuff method with photoelectric sensors (IITC Model 31 Computerized Blood Pressure Monitor, Life Sciences, Woodland Hills, CA, USA). The unit works with IITC hardware system to measure blood pressure and heart rate in conscious rats. The animals had been warmed in an enclosed chamber (IITC Model 303sc Animal Test Chamber IITC Life Sciences) for 30 min at ±30°C before taking BP readings. At the end of the 5-week experimental period, blood samples were also collected from all groups of animals by cardiac puncture into individual pre-cooled heparinized containers. Separated plasma was analyzed for Na+, K+, creatinine, and urea concentrations.
Determination of Proximal Tubule Na+ Handling
Male Sprague–Dawley rats were fed standard rodent chow supplemented with lithium chloride (12 mmol kg−1 dry weight) for 48 h prior to experimentation in order to raise plasma lithium to measurable concentrations without affecting renal sodium or water excretion.Citation16 The rats were prepared for acute renal clearance studies and MAP using a modified procedure that has been previously described.Citation3 Briefly, the rats were anesthetized by intraperitoneal injection of inactin [5-ethyl-5-(1′-methylpropyl)-2-thiobarbiturate, 0.11 g kg−1 body weight] (Sigma Aldrich, St. Louis, MO, USA) and tracheotomy was performed. The right jugular vein was cannulated to allow a continuous intravenous infusion of saline. A minimal abdominal incision was made, and a urinary bladder catheter was inserted for the collection of urine samples. The body temperature was maintained at 37 ± 1°C with a heated table throughout the experimental period. Immediately following the cannulation of the right jugular vein, a priming dose of creatinine (3 μg in 0.3 mL of 0.077 M NaCl) was given i.v. and the animals were placed on a continuous i.v. infusion of 0.077 M NaCl containing creatinine (0.15 μg mL−1) at 9 mL h−1 (Harvard Syringe Infusion Pump 22, Harvard Apparatus, Holliston, MA, USA). After a 3 h 30 min equilibration period, urine samples were taken every 30 min over the 4 h post-equilibration period of 1 h control, 1 h 30 min treatment, and 1 h 30 min recovery periods; blood samples were taken once per hour for the measurement of electrolyte and clearance marker concentrations. Animals were then divided into two groups that received either vehicle (0.077 M NaCl) or OA in 0.077 M NaCl at 90 μg h−1 for 1.5 h (treatment period). The infusate was then switched back to the vehicle for a further 1 h 30 min. Proximal tubular Na+ handling was assessed by the determination of endogenous lithium in plasma and urine and by the fractional excretion (FE) of lithium and sodium. For MAP measurements, a catheter was implanted in the left carotid artery for continuous recording of arterial blood pressure at 30 min intervals via a pressure transducer (Statham MLT 0380, Ad Instruments, Bella Vista, NSW, Australia), compatible with PowerLab System ML410/W (Bella Vista, NSW, Australia) and withdrawal of blood samples (0.3 mL). Plasma and urine samples were stored at 4°C until assay of creatinine, urea, and electrolytes was undertaken.
Analytic Procedures
Urine volume was determined gravimetrically. Na+ and K+ concentrations were determined by ion activity using the Beckman Coulter Counter (Synchron CX3 Clinical Systems, Fullerton, CA, USA). Urea and creatinine analyses were performed using the Beckman Coulter instrument. Creatinine estimation employed the reaction of creatinine and sodium picrate to form creatinine picrate. Urea estimation employed the hydrolytic degradation of urea in the presence of urease. The methods used reagent kits from Beckman Coulter, Ireland, Inc., and the concentrations were measured using Beckman Coulter (Synchron CX3 Clinical Systems).
Li+ was measured by flame emission spectrometry (Optima 2100 DV, Perkin Elmer, Shelton, CT, USA). The instrument was calibrated with Li+ working standards (0.16, 0.32, 0.64, 1.28, and 2.56 mg L−1) made from Li+ stock standard solution (5 mg L−1). Manually diluted (1:20) pool samples were then aspirated into the air/acetylene flame where neutral Li+ ions absorb light emitted from the Li+ hollow cathode lamp at 670.8 nm.Citation17 The standard solutions and samples were diluted in deionized water to suppress the interfering substances. FE of Na+ and Li+ was determined simultaneously.
Calculations
Lithium clearance (CLi) was used as a marker for the output of Na+ from the proximal tubules.Citation8 Renal clearances (C) and FE were calculated with the standard formulae where C = U × V/P and FE = 100 × C/GFR, where U is the urinary concentration, V is the urine flow rate, and P is the plasma concentration. FENa distal was calculated as CNa/CLi. GFR, the glomerular filtration rate, as assessed by creatinine clearance (CCr) was calculated using the standard formulae from measurements of the plasma and urinary concentrations of creatinine and urine flow rate in the fifth week.
Cell Viability Studies
Cell culture
The HEK293 cell line was obtained from the University of the Witwatersrand Medical School. HepG2 cells and MDBK cells were purchased from Highveld Biological Pty Ltd., Kelvin, South Africa. All cell lines were initially propagated in 25 cm2 flasks (Bibby Sterilin, Stone, Staffordshire, UK), at 37°C in EMEM (Gibco BRL, Inchinnan, Scotland) (5 mL) containing 10% (v/v) fetal bovine serum, 20 mM HEPES, 10 mM NaHCO3, 100 U mL−1 penicillin G, and 100 μg mL−1 streptomycin sulfate (Whittaker Bioproducts, Walkersville, MD, USA) at pH 7.5. Cells were divided 1:3 every 3–4 days and stored in a biofreezer (−80°C) in complete medium containing 10% DMSO.
MTT assay
The sensitivity of cells to the activity of OA was determined using a standard spectrophotometric 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.Citation18 Cells were trypsinized and seeded into 48-well plates (Bibby Sterilin) at a seeding density of 1.8 × 10Citation4 cells/well and incubated for 24 h to permit attachment and growth of cells to semi-confluency.
Thereafter, the medium (0.5 mL) containing the cells was changed and various doses of OA (0, 5, 10, 20, 40, and 80 μmol L−1) were added to the wells and cells incubated at 37°C for 24, 48 and 72 h. After each incubation period, the medium was removed and 200 μL of MTT in phosphate-buffered saline (5 mg mL−1) was added to the wells. The cells were incubated for 4 h to allow for the formation of blue formazan crystals. The MTT solution was then replaced with DMSO (200 μL/well) and absorbance was measured at 570 nm in a UV–visible spectrophotometer (Thermoscientific Biomate, Cambridge, UK). The percentage cell viability was then calculated as follows: [A570 treated cells – background]/[A570 control cells – background] × 100.
Single-cell gel electrophoresis (comet) assay
The single-cell gel electrophoresis (comet) assay is a simple method for measuring deoxyribonucleic acid (DNA) strand breaks in eukaryotic cells.Citation19,20 HEK293 and MDBK cells were seeded into 24-well plates (Bibby Sterilin) and incubated for 24 h to permit attachment and growth to semi-confluency. Thereafter, the cells were prepared as for MTT studies, but only two doses of OA (20 and 80 μmol L−1) were used. Preliminary studies indicated that all doses of OA (5–80 mmol L−1) used in the study did not induce any damage on the DNA; hence, comet assay studies were conducted with median and highest concentrations, that is, 20 and 80 μmol L−1, respectively. Frosted microscope slides were each covered with 400 μl of 1% normal melting point agarose in Ca2+- and Mg2+-free Kenny’s solution (0.4 M NaCl, 9 mM KCl, 0.7 mM K2HPO4, and 2 mM NaHCO3) and a coverslip was placed over the agarose, which was then allowed to solidify. The coverslips were removed and approximately 1.8 × 104 cells were added to 175 μL of 0.5% low melting point agarose in Kenny’s solution. The last layer of 200 μL of 1% normal melting point agarose was then added. The coverslips were removed, and the slides were placed in ice cold, freshly prepared lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, 1% Triton X-100, and 10% DMSO, pH 10) for 1 h. Thereafter, the slides were subjected to electrophoresis for 35 min at 25 V (0.83 V cm−1) in a BioRad horizontal gel electrophoresis unit (BioRad, Richmond, CA, USA) containing chilled electrophoresis buffer (300 mM NaOH and 1 mM EDTA, pH 13.5). Slides were washed with three changes of neutralization buffer (0.4 M Tris, pH 7.5), each for 5 min, to remove alkali and detergents. Slides were then stained with ethidium bromide (50 μL of a 20 μg mL−1 solution) and a coverslip was placed over the agarose. Slides were viewed using an Olympus inverted fluorescent microscope with a CC12 fluorescent camera (excitation filter of 450–490 nm and a barrier filter of 520 nm) (Wirsam Scientific & Precision Eq. LTD, Johannesburg, South Africa) which allowed for computerized image analysis for determination of DNA tails, linearly related to the frequency of DNA strand breaks.Citation21
Statistical analysis
Data are presented as the mean ± standard error of mean (SEM). Statistical comparison of the differences between the control and experimental groups was performed with GraphPad InStat Software (version 5.00, GraphPad Software, San Diego, CA, USA), using one-way analysis of variance, followed by Tukey–Kramer multiple comparison test. A value of p < 0.05 was considered significant.
RESULTS
Structure of OA
The data from 1H- and 13C-NMR (1D and 2D) spectroscopic analyses of the white powder obtained after recrystallization of the EAS with ethanol were comparable with literature data.Citation3,22
Renal Fluid and Electrolyte Excretion
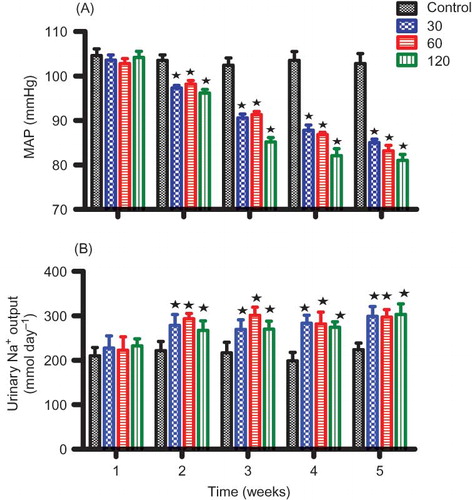
and compare the effects of OA on urinary Na+ outputs in groups of anesthetized and conscious rats with their respective control animals. Acute infusion (1 h 30 min) of OA (90 μg h−1) in anesthetized animals increased urinary Na+ excretion rate from the pre-treatment value of 713 to 847 μmol h−1 by the end of the treatment period (). On the other hand, sub-chronic administration (5 weeks) of various doses of OA (30, 60, and 120 mg kg−1, p.o.) twice every third day significantly (p < 0.05) increased urinary Na+ outputs in a dose-independent manner from week 2 to week 5 (). However, OA treatment did not change the urine flow and K+ and Cl− excretion rates of both conscious and anesthetized animals (data not shown).
Figure 1. Comparison between the effects of OA infusion in anesthetized rats with control animals on GFR (A), MAP (B), and Na+ excretion rate (C). OA was administered at 90 μg h−1 for 1 h 30 min during the treatment period. Values are presented as means for each 30-min collection; vertical bars indicate SEM of means (n = 6) in each group.
Note: *p < 0.05 in comparison with control animals.
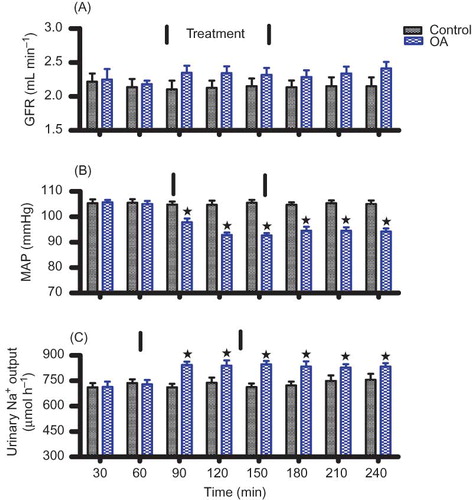
Figure 2. Comparison between the effects of OA treatment twice every third day for 5 weeks with control animals on MAP (A) and Na+ excretion rates (B) in conscious rats. Values are presented as means for weekly collection; vertical bars indicate SEM of means (n = 6) in each group.
Note: *p < 0.05 in comparison with control animals.
MAP and GFR Measurements
The GFR of control and OA-infused animals remained steady over the 4 h experimental period and did not statistically differ during the treatment (). OA administration for 1 h 30 min to anesthetized rats, however, significantly (p < 0.05) reduced MAP from a pre-treatment value of 105 to 92 mm Hg at the end of the treatment period (n = 6) without influencing the GFR (). The MAP of conscious animals was significantly (p < 0.05) decreased by all doses of OA from week 2 until the end of the 5-week experimental period (). OA also decreased significantly (p < 0.05) the plasma creatinine concentration of conscious animals with a concomitant increase in GFR by the end of the 5-week experimental period, but plasma urea concentration was not altered (). Kidney mass was not altered by sub-chronic OA treatment ().
Table 1. Terminal plasma biochemical parameters of control and rats administered OA twice every third consecutive day for 5 weeks (n = 6 in all groups).
Renal Clearance Measurements
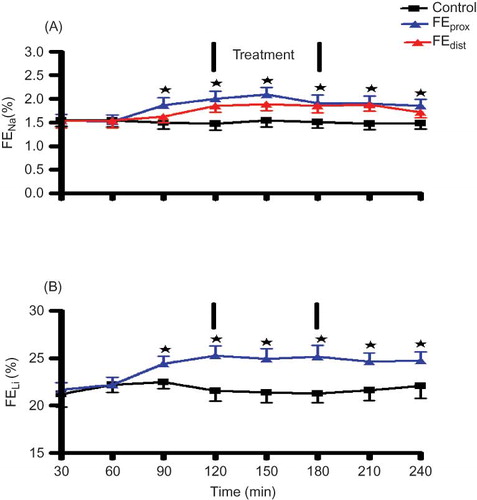
Lithium clearance (CLi) was used as a marker for proximal tubular sodium clearance.Citation23 The lithium doses used resulted in plasma lithium concentrations between 0.2 and 0.3 mmol L−1 with no difference between the groups. FELi and FENa did not differ between the control and the experimental groups prior to the infusion of OA (). The infusion of OA for 1 h 30 min significantly increased FELi, FENa prox, and FENa dist in comparison with control animals at the corresponding time (). However, OA infusion slightly reduced the FENa distal in comparison with FENa prox to levels that did not achieve statistical significance at the corresponding time period. In all cases, the FENa was not accompanied by any changes in FEK and FECl (data not shown).
Figure 3. Comparison between the effects of OA infusion in anesthetized rats with control animals on FENa prox, FENa dist (A) and FELi (B). OA was administered at 90 μg h−1 for 1 h 30 min during the treatment period. Values are presented as means, and vertical bars indicate SEM (n = 6 in each group).
Note: *p < 0.05 in comparison with control animals.
Cell Culture Studies
MTT assay
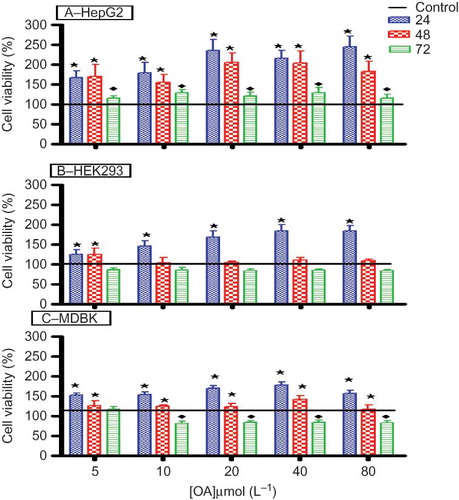
shows the percentage cell viability of HepG2, HEK293, and MDBK cell lines. The viability and/or metabolic activity of HepG2 cells increased significantly (p < 0.05) after 24- and 48-h exposure periods when compared to the control. However, the cell viability and/or metabolic activity decreased significantly (p < 0.05) after the 72-h exposure period in comparison to 24- and 48-h exposure periods, but remained above that of the control. OA treatment significantly increased (p < 0.05) the viability and/or metabolic activity of HEK293 and MDBK in a dose-dependent manner after 24-h exposure in comparison to both the control and the longer exposure periods. However, there was a slight decrease in the viability and/or metabolic activity of both kidney cell lines after 48- and 72-h exposure, which did not reach statistical significance when compared to the control.
Figure 4. The effects of OA on the viability and/or metabolic activity of HepG2 (A), HEK293 (B), and MDBK (C) cells in vitro after exposure to various concentrations of OA for 24, 48, and 72 h. Values are presented as means, and vertical bars indicate SEM (n = 6).
Notes: *p < 0.05 in comparison with control. ♦p < 0.05 in comparison with 24 and 48 h.
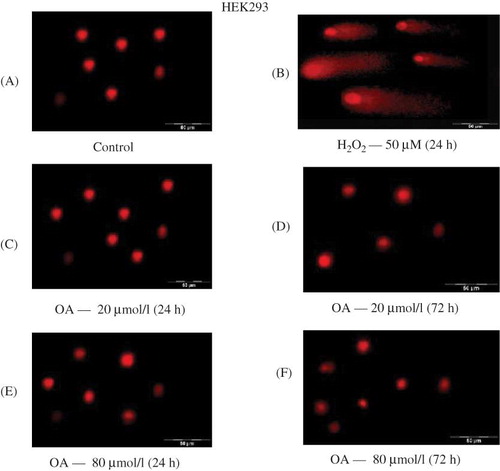
DNA integrity
shows the effects of OA on DNA integrity of HEK293 and MDBK cell lines, respectively, as assessed by the comet assay. Figure 5A shows no apparent DNA damage of control cells that were only suspended in the growth medium whilst Figure 5B indicates DNA damage as indicated by the comet tails in positive control cells treated with hydrogen peroxide (H2O2). H2O2 is considered to be an important reactive oxidant species and causes cell death via either apoptosis or necrosis.Citation24 HEK293 cells remained intact following exposure for 24 and 72 h to OA at concentrations of 20 and 80 μmol L−1, indicating that OA does not cause DNA damage in kidney cell lines. Similar results were obtained for the MDBK cells (results not shown).
DISCUSSION
The results of the present study apart from confirming our previous observations of the natriuretic effects of OA in ratsCitation3 suggest that this increased urinary Na+ output is in part mediated via the inhibition of proximal tubular Na+ reabsorption by the triterpene. Renal CLi, which has been widely used in animal studies and clinical investigations to assess proximal tubular function in the mammalian kidney,6,7,25–28 was used to estimate changes in proximal tubule Na+ reabsorption. The technique is based on the assumptions that Li+ is reabsorbed in the proximal tubules to the same degree as Na+.Citation29 Thus, a change in FELi estimates Na+ amount leaving the proximal tubule if GFR is unchanged. However, this is a controversial technique as there is evidence that lithium can be reabsorbed by the distal nephron under certain conditions such as Na+ and K+ depletion.10 It is reasonable to assume that CLi is a valid marker of proximal tubule function in this study, since the animals were Na+ and K+ replete. Therefore, any coincidental proximal tubular action of OA was associated with predominant secretion of Na+.
Specifically, OA infusion increased FENa and FELi, likely reflecting an inhibition of proximal tubular epithelial Na+ reabsorption. Indeed, the pronounced increase in urinary Na+ excretion rate evoked by OA infusion in the absence of significant changes in GFR suggests that OA inhibits proximal tubular reabsorption. The proximal tubule reabsorbs approximately two-thirds of the NaCl that enters the tubular fluid by glomerular filtrationCitation30 mediated by transport proteins.Citation31–33 The proximal tubule Na+ transporters on which OA exerts inhibitory action could not be identified by the current observations, nor is it clear whether OA has a direct effect on epithelial transport or works through an intermediary. Na+ is mainly reabsorbed across the proximal tubular cell in a process mediated by proximal tubule apical NHE3 followed by uphill active extrusion via basolateral Na+/K+ ATPase.Citation34,35 The literature on natriuretic hormones’ actions in the proximal tubule, however, shows that transport inhibition is effected via parallel independent inhibition of apical NHE3 and basolateral Na+/K+ ATPase.Citation36 We, therefore, speculate that OA inhibited apical NHE3 and basolateral Na+/K+ ATPase accounting for Na+ excretion since NHE3 is a major Na+ transport pathway in the renal proximal tubule.Citation31 Therefore, the reduction of FENa dist by OA to values that were below FENa prox, but statistically insignificant may be of biological importance compensating for changes in distal tubule sodium handling.
The GFR of anesthetized rats remained constant and the plasma concentration of electrolytes was unchanged in anesthetized rats following OA infusion, while sub-chronic treatment of conscious animals elevated the GFR with a concomitant reduction in plasma creatinine concentration. The current study cannot explain this discrepancy, but this may be attributed to the differences in the experimental protocols. In the anesthetized rats, GFR was assessed during the 1 h 30 min OA infusion period, while the GFR of conscious rats was assessed at the end of 5 weeks following oral administration of OA twice every third day. The OA-evoked increases in urinary Na+ excretion in conjunction with reduced plasma creatinine concentration and an elevated GFR over the 5-week experimental period suggest that these changes were in part mediated via enhanced renal plasma flow. The blood pressure lowering properties and amelioration of kidney function by OA confirm previous observations in STZ-induced diabetic rats.Citation37
The results further suggest that OA-evoked increase in Na+ excretion was not due to toxicity of this compound as shown by the MTT and comet assays. The MTT assay indicated that cell viability was significantly increased after 24 h, but long periods of exposure (48 and 72 h) of kidney cell lines to OA led to a progressive decrease in cell viability. The increased cell viability above that of the control could be as a result of a stimulatory effect on the cells by the OA, leading to increased cell proliferation in a time- and dosage-independent manner. Similar findings were obtained by other researchers.Citation38,39 We speculate that the decrease in cell viability with longer exposure periods could be partly attributed to the depletion of nutrients in the culture medium, which was not changed for the duration of the experimental period. This interpretation is supported by the observation that the control cells exhibited the same trend, indicating that the decrease was not totally drug induced. This was supported by the results of the comet assay where the treated cells lacked noticeable DNA tails which are clearly observed for the positive control. The comet assay is a simple and rapid technique to measure DNA damage or repair and genotoxicityCitation19,20 in individual cells. Undamaged DNA retains a highly organized association with matrix proteins in the nucleus, while this organization is disrupted in damaged cells. The concentrations of OA used in the current study (5–80 μmol L−1) compared with those previously used in other in vitro studies for the triterpene and crude extracts containing such compounds.Citation40–42
Kidney structure is affected in diabetes due to glomerular hypertrophy caused by accumulation of extracellular matrix proteins and injury of the epithelial cells.Citation43,44 We have, however, reported that extracts of Persea americana Mill (Lauraceae) (“Avocado”) decrease the viability of kidney cell lines in vitro.Citation45 Furthermore, aristolochic acid isolated from Aristolochia fangchi was shown to induce cellular injury and apoptosis in proximal tubular (LLC-PK1) epithelial cell lines.Citation46–49 The results described in this study introduce the first in vivo evidence that the previously reported OA-evoked increases in urinary Na+ output are in part mediated via inhibition of Na+ reabsorption and increases in proximal tubular Na+ secretion. The study provides the first in vitro evidence that OA does not exhibit toxicity in kidney and liver cell lines and implicates the proximal tubule in the natriuretic effects of OA.
ACKNOWLEDGMENTS
This study was partly funded by the University of KwaZulu-Natal, Research Division. The authors are grateful to the Biomedical Research Unit, University of KwaZulu-Natal, for the supply of animals.
Declaration of interest: The authors declare that there is no interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49(2):57–68.
- Fai YM, Tao CC. Literature review on pharmaceutical activities of oleanolic acid. Natura Proda Medica. 2009;2:291–298.
- Mapanga RF, Tufts MA, Shode FO, Musabayane CT. Renal effects of plant-derived oleanolic acid in streptozotocin-induced diabetic rats. Ren Fail. 2009;31(6):481–491.
- Musabayane C, Tufts MA, Mapanga RF. Synergistic antihyperglycemic effects between plant-derived oleanolic acid and insulin in streptozotocin-induced diabetic rats. Ren Fail. 2010;32:832–839.
- Musabayane CT, Ndhlovu CE, Balment RJ. Renal fluid and electrolyte handling in streptozotocin-diabetic rats. Ren Fail. 1995;17(2):107–116.
- Thomsen K. Lithium clearance as a measure of sodium and water delivery from the proximal tubules. Kidney Int. 1990;37(28):10–16.
- Shirley D, Walter SJ. Current status of the lithium clearance method for the assessment of renal proximal tubular function. Lithium. 1993;4:25–31.
- Thomsen K, Shirley DG. The validity of lithium clearance as an index of sodium and water delivery from the proximal tubules. Nephron. 1997;77:125–138.
- Atherton JC, Doyle A, Gee A, . Lithium clearance: Modification by the loop of Henle in man. J Physiol. 1991;437:377–391.
- Shirley DG, Walter SJ. Renal tubular lithium reabsorption in potassium-depleted rats. J Physiol. 1997;501(3):663–670.
- Bevevino LH, Aires MM. Effect of crude extract of roots of Bredemeyera floribunda Willd. II. Effect on glomerular filtration rate and renal tubular function of rats. J Ethnopharmacol. 1994;43(3):203–207.
- Kreydiyyeh SI, Usta J. Diuretic effect and mechanism of action of parsley. J Ethnopharmacol. 2002;79(3):353–357.
- Li A, Bode C, Sakai Y. A novel in vitro system, the integrated discrete multiple organ cell culture (IdMOC) system, for the evaluation of human drug toxicity: Comparative cytotoxicity of tamoxifen towards normal human cells from five major organs and MCF-7 adenocarcinoma breast cancer cells. Chem-Biol Interact. 2004;150:129–136.
- Cummings BS, Zangar RC, Novak RF, Lash LH. Cytotoxicity of trichloroethylene and S-(1,2-dichlorovinyl)-L-cysteine in primary cultures of rat renal proximal tubular and distal tubular cells. Toxicology. 2000;150:83–98.
- Musabayane CT, Mahlalela N, Shode FO, Ojewole JAO. Effects of Syzygium cordatum (Hochst). [Myrtaceae] leaf extract on plasma glucose and hepatic glycogen in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;97:485–490.
- Shalmi M, Thomsen K. Alterations of lithium clearance in rats by different modes of lithium administration. Ren Physiol Biochem. 1989;12:273–280.
- Gan K, Lin CN. Studies on the constituents of Formossan gentianaceous plants. XI. Constituents of Gentiana flavormaculata and Tripterrospermum taiwanense and the antihepatotoxic activity of ursolic acid derivatives. Chin Pharmaceut J. 1988;40:77–84.
- Mossman T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
- Singh N, McCoy M, Tice R, Schneider E. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191.
- Anderson D, Plewa MJ. The International Comet Assay Workshop. Mutagenesis. 1998;13:67–73.
- McKelvey-Martin VJ, Ho ETS, McKeown SR, . Emerging applications of the single cell gel electrophoresis (comet) assay. I. Management of invasive transitional cell human bladder carcinoma. II. Fluorescent in situ hybridisation comets for the identification of damaged and repaired DNA sequences in individual cells. Mutagenesis. 1998;13:1–8.
- Mahato SB, Kundu AP. 13C NMR spectra of pentacyclic triterpenoids—a complication and some salient features. Phytochemistry. 1994;37:1517–1573.
- Thomsen K, Shirley DG. A hypothesis linking sodium and lithium reabsorption in the distal nephron. Nephrol Dial Transplant. 2006;21:869–880.
- Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soc Trans. 2001:29(2): 345–350.
- Koomans H, Boer WH, Dorhout Mees EJ. Evaluation of lithium clearance as a marker of proximal tubule sodium handling. Kidney Int. 1989;36(1):2–12.
- Walter SJ, Shirley DG. Effect of frusemide on lithium clearance and proximal tubular reabsoption in anaesthetized rats. J Physiol. 1991;437:85–93.
- Boer WH, Fransen R, Shirley DG, Walter SJ, Boer P, Koomans HA. Evaluation of the lithium clearance method: Direct analysis of tubular lithium handling by micropuncture. Kidney Int. 1995;47:1023–1030.
- Whiting P. The use of lithium clearance measurements as an estimate of glomerulo-tubular function. Ren Fail. 1999;21(3–4): 421–426.
- Leyssac PP, Christensen P. A comparison between endogenous and exogenous lithium clearance in the anaesthetized rat. Acta Physiol Scand. 1994;151:173–179.
- Aronson P. Red-cell sodium–lithium countertransport and essential hypertension. N Engl J Med. 1982;307:317.
- Aronson PS. Ion exchangers mediating NaCl transport in the renal proximal tubule. Cell Biochem Biophys. 2002;36(2–3):147–153.
- Girardi A, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol. 2008;294:F414–F422.
- Yingst D, Araghi A, Doci TM, Mattingly R, Beierwaltes WH. Decreased renal perfusion rapidly increases plasma membrane Na-K-ATPase in rat cortex by an angiotensin II-dependent mechanism. AJP - Renal Physiol. 2009;297(5):F1324–F1329.
- Lorenz J, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol Renal Physiol. 1999;277:F447–F453.
- Magyar CE, Zhang Y, Holstein-Rathlou NH, McDonough AA. Proximal tubule Na transporter responses are the same during acute and chronic hypertension. Am J Physiol Renal Physiol. 2000;279:F358–F369.
- Zhang Y, Mircheff AK, Hensley CB, . Rapid redistribution and inhibition of renal sodium transporters during acute pressure natriuresis. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;270:F1004–F1014.
- Mapanga R, Tufts MA, Shode FO, Musabayane CT. Renal effects of plant-derived oleanolic acid in streptozotocin-induced diabetic rats. Ren Fail. 2009;31:481–491.
- Yeap SK, Alitheen NBM, Yong Ho W, . Immunomodulatory role of Rhaphidophora korthalsii methanol extract on human peripheral blood mononuclear cell proliferation, cytokine secretion and cytolytic activity. J Med Plants Res. 2010;5(6):958–965.
- Akhir NAM, Chua LS, Majid FAA, Sarmidi MR. Cytotoxicity of aqueous and ethanolic extracts of Ficus deltoidea on human ovarian carcinoma cell line. Br J Med Med Res. 2011;1(4):397–409.
- Li J, Guo W-J, Yang Q-Y. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J Gastroenterol. 2002;8(3):493–495.
- Emília JM, Uwe W, Ruiz-Gutierrez V, Daniel H, Planas JM. Olive fruit extracts inhibit proliferation and induce apoptosis in HT-29 human colon cancer cells. J Nutr. 2006;136(10):2553–2557.
- Sung HY, Kang SW, Kim JL, . Oleanolic acid reduces markers of differentiation in 3T3-L1 adipocytes. Nutr Res. 2010;30(12):831–839.
- Yamagishi S, Inagaki Y, Okamoto T, Amano S, Koga K, Takeuchi M. Advanced glycation end products inhibit de novo protein synthesis and induce TGF-b overexpression in proximal tubular cells. Kidney Int Suppl. 2003;63:464–473.
- Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–233.
- Gondwe M, Kamadyaapa DR, Tufts M, Chuturgoon AA, Musabayane CT. Sclerocarya birrea [(A. Rich.) Hochst.] [Anacardiaceae] stem-bark ethanolic extract (SBE) modulates blood glucose, glomerular filtration rate (GFR) and mean arterial blood pressure (MAP) of STZ-induced diabetic rats. Phytomedicine. 2008;15:699–709.
- Vanhaelen M, Vanhaelen-Fastre R, But P, Vanherweghem JL. Identification of aristolochic acid in Chinese herbs. Lancet. 1994;343:174.
- Lebeau C, Arlt VM, Schmeiser HH, . Aristolochic acid impedes endocytosis and induces DNA adducts in proximal tubule cells. Kidney Int. 2001;60:1332–1342.
- Balachandran P, Wei F, Lin R, Khan IA, Pasco DS. Structure activity relationships of aristolochic acid analogues: Toxicity in cultured renal epithelial cells. Kidney Int. 2005;67:1797–1805.
- Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: A worldwide problem. Int Society Nephrol. 2008;74:158–169.