Abstract
Background: Methylmalonic aciduria is an inborn error of metabolism that causes renal failure and tubulointerstitial (TI) nephritis as complications. This study aimed to examine the levels of expression of several genes related to inflammation, oxidative stress, and mitochondrial function in the renal cortex of rats receiving methylmalonic acid (MMA). Methods: Rats received MMA subcutaneously for a month. Tumor necrosis factor alpha (TNFα), nuclear factor-kappa B, interleukin 1 beta (IL-1β), and cyclooxygenase 2 (COX-2) genes were examined by real-time polymerase chain reaction. We also examined transforming growth factor beta (TGF-β) related to TI fibrosis, c-FOS, belonging to the immediate early gene family of transcription factors, and expression of SIRT1, related to energy production. Results: There was significantly higher expression of TNFα and a trend toward a higher level of TGF-β transcripts in the methylmalonic model group compared with the controls. However, SIRT1 expression was not different among the groups. Urinary MMA excretion correlated positively with mRNA level of TGF-β. The expression of COX-2 was positively associated with the expression of c-FOS and inversely related to the expression of IL-1β. Conclusions: The higher levels of TNFα and TGF-β transcripts suggest inflammation and differentiation processes in the renal cortex in rats because of MMA. After 1 month of MMA injections, expression levels of SIRT1 were not affected, suggesting mitochondrial preservation in early stages of the disease.
INTRODUCTION
Methylmalonic acidemias or acidurias (OMIM 251000) are a heterogeneous group of inborn errors of metabolism of branched-chain amino acids, odd-numbered fatty acids, and cholesterol, leading to the accumulation of methylmalonic acid (MMA) in urine and other body fluids. They are caused by an impairment in the mitochondrial enzyme methylmalonyl-CoA mutase (MUT) (EC 5.4.99.2) or by a defect in the uptake, transport, or synthesis of cobalamin, the cofactor of MUT.Citation1 MUT defects caused by mutations in the mutase apoenzyme locus are further subdivided into defects without residual activity (mut0) and defects with residual activity (mut−). Patients with methylmalonic aciduria develop progressive renal failure as one of the most serious complications.Citation2 This fact merits special attention, since the complication of renal failure is particular to methylmalonic aciduria, and is not observed in other organic acidurias, not even in propionic aciduria, caused by a deficiency in the aforementioned enzyme of the same catabolic pathway. Indeed, the physiopathological basis for the development of renal failure remains unclear.Citation3 The small number of examinations of pathological kidneys in these patients reported in the literature presents a fairly uniform picture of tubulointerstitial (TI) nephritis with interstitial fibrosis, tubular atrophy, and mononuclear inflammatory infiltrate.Citation4
Previous studies have demonstrated that bovine serum albumin-loaded proteinuric rats developed TI injury in the renal cortex via an activation of nuclear factor-kappa B (NF-κB), one of the key transcriptional factors in the regulation of proinflammatory gene expression and apoptosis.Citation5,6 In addition, gene transfer of the truncated form of IκB alpha by a recombinant adenovirus vector, with specific inhibition of NF-κB activation, attenuated these changes in the proximal tubules.Citation7 These results suggest that NF-κB-inducible genes play an important role in this model of TI injury. A recently published review on the physiopathology of TI lesions concludes that proinflammatory mechanisms, oxidative stress, vascular problems, and the presence of proteinuria are important factors in the onset and development of TI lesions.Citation8 In patients with methylmalonic acidemia, mitochondrial function is found to be impaired,Citation9 as is the expression of SIRT1, which produces the protein sirtuin 1Citation10 and is related to the energy status of the cell. Therefore, the aim of our study is to assess the expression levels of genes related to inflammation and oxidative stress [tumor necrosis factor alpha (TNFα), NF-κB, interleukin 1 beta (IL-1β), and cyclooxygenase 2 (COX-2)], cell proliferation and fibrosis [transforming growth factor beta (TGF-β) and c-FOS], and mitochondrial function (SIRT1) in the renal cortex of rats administered MMA. As a secondary aim, we considered that it would be interesting to evaluate the effect of pentoxifylline, due to its ability to inhibit TNFα.Citation11,12
MATERIALS AND METHODS
Animals and Experimental Design
Adult male Wistar rats {[CRL: (WI) BR] Criffa, Barcelona, Spain}, weighing 200–250 g, were used in this study. Rats were housed individually in metabolic cages which were placed in an air-conditioned room (18–22°C) with a light/dark cycle of 12 h. The study was approved by the Ethics Committee of Cruces Hospital. Animal studies were carried out in compliance with American National Institutes of Health (NIH) Principles of Laboratory Animal Care (NIH Publication 25, no. 28, revised 1996) and with the European guidelines for the care of laboratory animals (86/609/EEC).
Animals were randomly assigned to one of the following five groups:
Control: Animals were not subjected to any kind of manipulation, and were housed under the same conditions as the rest.
Simulation: These animals received injections at the same frequency as the rats in the following three groups but in this case containing only saline solution.
Low MMA model: Rats received subcutaneous injections of solutions of MMA in carbonate/bicarbonate buffer at pH 7.4. MMA injections were administered twice a day at the following doses: 0.72 μmol/g for the first 8 days; 0.89 μmol/g for the following 8 days; and finally, 1.67 μmol/g until the 28th day.Citation13
High MMA model: The same protocol as the previous group, but injecting twice the amount of MMA.
PTX: Rats received the same amount of MMA as the High MMA model and, in addition, 150 mg/kg/day of pentoxifylline everyday in a single dose.
Rats had free access to water and standard laboratory food (maintenance chow A04, Panlab, Barcelona, Spain) with the following composition per kg consumed:
| • | Proteins 172 g | ||||
| • | Fat 27 g | ||||
| • | Carbohydrates 597 g | ||||
| • | Minerals 44 mg | ||||
| • | Dietary fiber 39 g | ||||
| • | Water content 120 g | ||||
| • | Caloric value 3100 kcal | ||||
Measurement of Metabolites of MMA in Urine
Urine was collected for 12 h before the animals were killed. Organic acids were extracted from acidified urine with ethyl acetate. The extract was evaporated to dryness and derivatized to produce the volatile trimethylsilyl esters. Acids were analyzed using gas chromatography–mass spectrometry in the single ion monitoring mode.Citation14
Extraction of Total RNA
Total RNA was extracted from the renal cortex by the TRIzol reagent method (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and after homogenization of the tissue in a TissueLyser (Qiagen, Valencia, CA, USA). The RNA obtained was quantified in triplicate using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The integrity and purity of the RNA was verified using spectrophotometry and the Bioanalyzer system (Agilent Technologies, Madrid, Spain). Only samples showing high quality and integrity [RNA integrity number (RIN) above 6] were considered eligible for the next stage of the experiment.
Subsequently, cDNA was synthesized from total RNA using a Multiplex RT for TaqMan array kit, as described by the supplier (Applied Biosystems, Foster City, CA, USA).
Quantitative Real-Time Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (RT-PCR) was performed to investigate the expression levels of the genes using the TaqMan quantitative RT-PCR assay. Quantitative RT-PCR was performed using the 7900 HT Fast Real-Time PCR System (Applied Biosystems). TaqMan probes for genes were also obtained from Applied Biosystems.
Expression levels of all transcripts were determined relative to an internal housekeeping gene. Several different genes [glyceraldehydes 3-phosphate dehydrogenase (GADPH), β-actin, and 18S] were tested as possible housekeeping genes. Of these, the β-actin control gene was selected, because it did not show altered expression in any of the studied treatments. Expression levels of all transcripts were determined in triplicate.
Statistical Analysis
The fold change (2−ΔΔ Ct) in the target gene, normalized to β-actin and relative to the expression of control group, was calculated for each sample according to the manufacturer’s guidelines (Applied Biosystems).Citation15
Kolmogorov–Smirnov and Shapiro–Wilk tests were used to determine the distribution of the data. Differences in clinical characteristics and mRNA gene expression among the groups were assessed by Kruskal–Wallis test, whereas Mann–Whitney U test was used to assess differences between each group and the control group. Bivariate associations between urinary excretion of methylmalonic and methylcitric acids, and creatinine and gene expression levels were assessed using Spearman’s correlation analysis. Data are presented as the median and range unless otherwise indicated. Statistical significance was set at p < 0.05. The statistical analysis was carried out using PASW Statistics (version 18) (SPSS Inc., Chicago, IL, USA).
RESULTS
After 28 days of treatment, there were no differences in rat kidney or total body weight or in urinary methylcitric acid between the groups. As expected, the concentration of urinary MMA measured in the three groups of rats receiving MMA was significantly higher than in the control and simulation groups ().
Table 1. Body weight gain and metabolic profile in rats after 28 days of MMA injections.
The mRNA expression levels of TNFα, NF-κB, IL-1β, COX-2, TGF-β, c-FOS, and SIRT1 genes were analyzed and compared with the control group (). The control and the simulation group did not differ in the expression of any of the genes analyzed. However, TNFα transcript levels were elevated in the group that received the higher dose of MMA (p = 0.021) than in the control group (). Rats receiving the higher methylmalonic dose also tended to have a higher mRNA level of TGF-β (p = 0.095) compared with the control group. No significant differences in the pentoxifylline group were observed with respect to the control or the MMA groups for TNFα () or TGF-β (). On the contrary, there was a trend toward lower NF-κB expression in the pentoxifylline group than in the control group (p = 0.071). There were no significant differences in the expression of SIRT1 across all the groups.
Figure 1. Relative mRNA expression (geometric mean and standard error of the mean as range) of TNFα and statistical differences among control, simulation, low MMA, high MMA, and PTX groups.
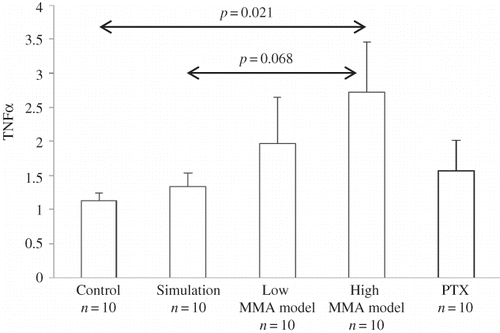
Figure 2. Graphical illustrations of the correlations found between (A) the expression of TGF-β and the urinary excretion of MMA, and (B) the expression of SIRT1 and TGF-β.
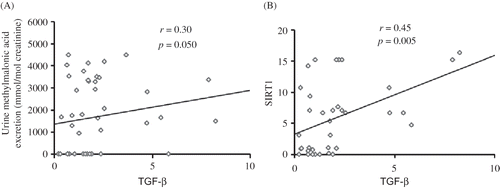
Table 2. Relative mRNA expression (median and range) of inflammatory and oxidative stress-related genes.
Urinary MMA excretion correlated positively with mRNA level of TGF-β (r = 0.30, p = 0.050) () and there was a positive correlation between levels of expression of SIRT1 and TGF-β mRNA (r = 0.45, p = 0.005). COX-2 mRNA expression was positively associated with c-FOX expression (r = 0.44, p = 0.018) and inversely related to IL-1β expression (r = −0.43, p = 0.011).
DISCUSSION
In this study, we have investigated the renal expression of several genes related to inflammation, oxidative stress, fibrosis, proliferation, differentiation, and mitochondrial function in rats receiving MMA subcutaneously. The higher levels of TNFα and TGF-β transcripts suggest inflammation and differentiation processes in the renal cortex of MMA rats. However, after 1 month of MMA injections, expression levels of SIRT1 were not affected, suggesting mitochondrial preservation in early stages of the disease.
Dysfunction of proximal tubules resulting in TI nephritis and chronic renal failure is a common long-term complication of methylmalonic acidemias. The risk of developing renal failure seems to be related to high levels of MMA over time and depends on the disease type. Patients with mutase deficiency (mut0 more than mut−) are at a higher risk than patients with deficiencies in the metabolism of cobalamin, especially if treatable with vitamin B12. In our case, we expected that a month of injecting MMA should be sufficient to cause observable renal damage. But the lack of differences in the values of methylcitric acid among groups was not indicative of any severe progression of methylmalonic acidemia, because reaction sequence to form methylcitrate by MMA injections is energetically unfavorable. Changes in gene expressions, however, led us to explore another theory in more depth.
It has been demonstrated that accumulating organic acids and CoA esters play a central role in the neuropathogenesis of methylmalonic acidemia. In particular, propionyl-CoA and methylcitric acid synergistically inhibit enzymes of the tricarboxylic acid cycle, pyruvate dehydrogenase complex, and respiratory chain,Citation16 whereas it has been suggested that MMA competes with the mitochondrial import of succinate.Citation17 In contrast to the neuropathogenesis, mechanisms underlying chronic renal failure have not yet been extensively studied. Clinically, progressive TI nephritis with mononuclear cell infiltration, interstitial fibrosis, and tubular atrophy has been observed.Citation18 Further, proximal and distal tubules of affected patients display functional abnormalities, including decreased reabsorption of phosphate, impaired acid–base balance, and reduced ability to concentrate urine, probably due to the presence of megamitochondria in tubular cells.Citation19
The participation of TI lesions in the loss of kidney function has been tacitly recognized since the development of kidney biopsy techniques made it possible to examine affected kidneys before the development of end-stage renal injury. Moreover, it was nearly four decades ago when Risdon et al.Citation20 reported that the severity of tubular damage had a more significant correlation with the reduction of creatinine clearance than glomerular damage scores.
Recently, a hypothetical model has been developed to explain the renal toxicity of MMACitation21 postulating that, physiopathologically, accumulating metabolites of this disease induce a dysfunction of mitochondrial energy production as well as dysfunction of transport processes in the proximal tubule. We know that knockout mice would be an ideal model for methylmalonic acidemia, but injection of MMA to healthy rats is not a limitation in this study, since we demonstrate that MMA per se could modify gene expression. Indeed, increased values of TNFα and TGF-β indicate that the presence of MMA can cause inflammation and oxidative stress, as it occurs in early stages of metabolic disease. Moreover, correlations among expressions of the various genes studied would be useful tools to predict renal consequences of methylmalonic acidemia such as oxidative stress and mitochondrial dysfunction. Previous animal and human data have shown how MMA induces oxidative stress in vivo in the brain and fibroblasts.Citation22
In order to understand the underlying physiopathology of TI damage, we have to bear in mind that increments in interstitial volume and reported fibrosis are correlated with renal functional impairment and worse prognosis. On the contrary, the decrease in the numbers of peritubular capillaries, the morphological changes in tubular epithelia, and the intensity of inflammatory infiltration in the interstitium have also been found to be correlated with renal functional deterioration.Citation23 Emerging evidence suggests that during TI fibrosis, the tubular epithelial cells are capable of transdifferentiation into fibroblasts/myofibroblasts, in a process known as tubular epithelial–myofibroblast transdifferentiation. In this process of renal fibrosis, TGF-β plays a critical role as our results show, the High MMA model presenting a significant increase in this factor.
Our group has provided evidence that there is a significantly higher expression of the gene related to inflammation, TNFα, in the rat receiving MMA. This fact suggests that inflammatory processes can play a role in the development of the TI lesion.Citation24 We should point out that the TGF-β levels are significantly related to the quantitative urinary excretion of MMA, suggesting the importance of this organic acid in the physiopathology of TI damage.Citation9 So, MMA levels could predict progression of renal damage, because its signaling may initiate proapoptotic effectors in tubular epithelial cells resulting in tubular degeneration and atrophy. These processes of fibrosis and apoptosis as putative pathogenic mechanisms for progressive nephron loss have important implications for future TI fibrosis and glomerulosclerosis. The importance of TGF-β in the progression of renal disease also revolves around the fact that TGF-β isoforms and bone morphogenetic proteins are widely expressed and act on every cell by engaging an intracellular signaling cascade of SMAD family proteins through ligand-induced activation of heteromeric transmembrane TGF-β receptor kinases. Their accumulation in the nucleus leads to transcriptional activation of target genes.Citation25
Emerging evidence points to a disturbance of cellular energy metabolism by MMA and related metabolites.Citation16 As the tubule cell has high energy requirements and TI nephritis can also be found in patients with primary mitochondrial disorders,Citation26 this might be a first physiopathological link. It is worth mentioning that although the differences we observed in the expression of SIRT1 between control and model rats were not significant, it is quite likely that they would have reached significance if the number of experiments performed or the time of exposure to the acid had been greater. In any case, mitochondrial dysfunction would take place at later stages of renal failure, and it is known to be positively correlated with inflammatory processes through TGF-β in early stages. Specifically, this statement is in accordance with the results obtained by other authors, whose studies with an MMA rat model revealed an initial increase in complex II–IV enzyme activity, followed by a decrease in complex II and III activity. These authors speculated that the initial increase in mitochondrial capacity may be a compensatory mechanism in response to the mitochondrial toxicity of accumulating metabolites, but later MMA interferes in the synthesis of cytochromes.Citation27
Recent studies have shown that TI injury induced by massive proteinuria plays an important role in the development of renal dysfunction and the progression of renal disease. Large amounts of proteins filtered stimulate renal tubular cells to produce proinflammatory cytokines and extracellular matrix proteins, which contribute to the progression of TI damage.Citation6 In our model of MMA, proteinuria was not detected in rats using urine test strips. This might explain why we did not observe differences in the expression of IL and COX-2, despite the two factors being inversely correlated; they are predictors of inflammation and oxidative stress acting in opposite ways. We find the correlation between COX-2 and c-FOX interesting, suggesting that cell proliferation, differentiation, and inflammation start at the same time as oxidative stress at early stages of renal disease in methylmalonic acidemia. It should also be taken into account that expression levels of genes related to these processes have been considered despite the lack of histological analysis, which we assume as a limitation of our study.
On the contrary, pentoxifylline might have a renoprotective effect due to the modulating effect on TNF level, which is independent of the concentration of MMA. In this work, we found a trend toward lower NF-κB expression in the pentoxifylline group, which would mean a lower inflammation and apoptosis. A number of in vitro experiments have assessed the blockade of TGF-β using antifibrotic agents, such as N-acetyl-l-cysteines as well as angiotensin-converting enzyme inhibitors or angiotensin II antagonists.Citation28,29 This fact and our results indicate possible therapeutic targets which could be vulnerable to the use of drugs such as pentoxifylline or angiotensin receptor blockers.Citation30
We can conclude that, according to our findings, TNFα associated to TGF-β could play a substantial role in inflammation and differentiation processes of renal damage in MMA acidemia, and that this is a physiopathological condition prior to mitochondrial dysfunction.
ACKNOWLEDGMENT
The authors thank the research network SAMID (Spanish Ministry of Health) for financial support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Fenton WA, Gravel RA, Rosenblatt DS. Disorders of propionate and methylmalonate metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001:2165–2193.
- Baumgarter ER, Viardot C. Long-term follow-up of 77 patients with isolated methylmalonic aciduria. J Inherit Metab Dis. 1995;18:138–142.
- Hörster F, Hoffmann GF. Pathophysiology, diagnosis, and treatment of methylmalonic aciduria—recent advances and new challenges. Pediatr Nephrol. 2004;19:1071–1074.
- Rutledge SL, Geraghty M, Mroczek E, . Tubulointerstitial nephritis in methylmalonic acidemia. Pediatr Nephrol. 1993;7:81–82.
- Zoja C, Donadelli R, Colleoni S, . Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kappaB activation. Kidney Int. 1998;53:1608–1615.
- Remuzzi G, Perna A, Benigni A. Proteins abnormally filtered throughout glomerular capillary have an intrinsic renal toxicity. Contrib Nephrol. 1996;118:164–172.
- Takase O, Hirahashi J, Takayanagi A, . Gene transfer of truncated IkappaBalpha prevents tubulointerstitial injury. Kidney Int. 2003;63:501–513.
- Rodríguez-Iturbe B, García G. The role of tubulointerstitial inflammation in the progression of chronic renal failure. Nephron Clin Pract. 2010;116:c81–c88.
- de Keyzer Y, Valayannopoulos V, Benoist JF, . Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr Res. 2009;66:91–95.
- Crujeiras AB, Parra D, Goyenechea E, . Sirtuin gene expression in human mononuclear cells is modulated by caloric restriction. Eur J Clin Invest. 2008;38:672–678.
- DiPetrillo K, Gesek FA. Pentoxifylline ameliorates renal tumor necrosis factor expression, sodium retention, and renal hypertrophy in diabetic rats. Am J Nephrol. 2004;24:352–359.
- Hoie EB, McGuire TR, Leuschen PM, Zach TL. Pentoxifylline inhibits tumor necrosis factor-alpha induced synthesis of complement component C3 in human endothelial cells. Biol Pharm Bull. 2004;27:1670–1673.
- Wajner M, Brites EC, Dutra JC, . Diminished concentrations of ganglioside N-acetylneuraminic acid (G-NeuAc) in cerebellum of young rats receiving chronic administration of methylmalonic acid. J Neurol Sci. 1988;85:233–238.
- Prieto JA, Andrade F, Aldámiz-Echevarría L, . Análisis de ácidos orgánicos en orina mediante cromatografía de gases-espectrometría de masas. Química Clín. 2007;26:66–72.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408.
- Kölker S, Schwab M, Hörster F, . Methylmalonic acid, a biochemical hallmark of methylmalonic acidurias but no inhibitor of mitochondrial respiratory chain. J Biol Chem. 2003;278:47388–47393.
- Mirandola SR, Melo DR, Schuck PF, . Methylmalonate inhibits succinate-supported oxygen consumption by interfering with mitochondrial succinate uptake. J Inherit Metab Dis. 2008;31:44–54.
- D’Angio CT, Dillon MJ, Leonard JV. Renal tubular dysfunction in methylmalonic acidemia. Eur J Pediatr. 1991;150:259–263.
- Chandler RJ, Zerfas PM, Shanske S, . Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J. 2009;23:1252–1261.
- Risdon RA, Sloper JC, de Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366.
- Morath MA, Okun JG, Müller IB, . Neurodegeneration and chronic renal failure in methylmalonic aciduria—A pathophysiological approach. J Inherit Metab Dis. 2008;31:35–43.
- Fernandes CG, Borges CG, Seminotti B, . Experimental evidence that methylmalonic acid provokes oxidative damage and compromises antioxidant defenses in nerve terminal and striatum of young rats. Cell Mol Neurobiol. 2011;31(5):775–785.
- Becker GJ, Hewitson TD. The role of tubulointerstitial injury in chronic renal failure. Curr Opin Nephrol Hypertens. 2000;9:133–138.
- Miana M, de Las Heras N, Rodriguez C, . Effect of eplerenone on hypertension-associated renal damage in rats: Potential role of peroxisome proliferator activated receptor gamma (PPAR-γ). J Physiol Pharmacol. 2001;62:87–94.
- Böttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610.
- Buemi M, Allegra A, Rotig A, . Renal failure from mitochondrial cytopathies. Nephron. 1997;76:249–253.
- Sauer SW, Opp S, Haarmann A, . Long-term exposure of human proximal tubule cells to hydroxycobalamin[c-lactam] as a possible model to study renal disease in methylmalonic acidurias. J Inherit Metab Dis. 2009;32:720–727.
- Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997;51:2–15.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: From ACEI to angiotensin II antagonists. Kidney Int. 2000;57:1803–1817.
- Ha TS, Lee JS, Hong EJ. Delay of renal progression in methylmalonic acidemia using angiotensin II inhibition: A case report. J Nephrol. 2008;21:793–796.