Abstract
Objectives: Several equations for the estimation of glomerular filtration rate (GFR) from serum cystatin C have been reported. We compared the results obtained using these equations to test the homogeneity of their results as well as their usefulness in clinical practice. Design and methods: Seven hundred and twenty-seven outpatients were studied. Of these, 439 were male and 288 were female, and their mean age was 60.8 ± 24.1 years. GFR was estimated from serum creatinine using the abbreviated Modification of Diet in Renal Disease (MDRD-4) equation. GFR was estimated from serum cystatin C levels using five different equations. Results: The simplest (100/cystatin C) formula rendered the highest estimated GFR and the Hoek’s equation rendered the lowest GFR, even significantly lower than the MDRD-4 equation (p < 0.001, Student’s t-test). From the simplest formula to the Hoek equation the mean difference calculated was 25.1 ± 8.7 mL/min (p < 0.001, Student’s t-test). No differences by gender were found among the results of different equations. All cystatin C-derived equations reduced the number of patients diagnosed of chronic renal failure when compared with MDRD-4 formula. No patient with normal renal function was shifted to the renal disease group. Conclusions: A higher value could be expected when GFR is estimated from cystatin C. Nevertheless, vast differences were found in the results when tested using several equations. Physicians should be aware of this problem to avoid a wrong clinical diagnosis of renal function.
INTRODUCTION
Over the past decades several different markers for the estimation of glomerular filtration rate (GFR) have been proposed. The ideal marker for the estimation of GFR should be an endogenous molecule produced at a constant rate, which is cleared solely by the kidneys via free glomerular filtration, without being secreted either by tubular cells or reabsorbed into peritubular circulation.Citation1 The “gold standard” for the estimation of GFR is a clearance of exogenous substances such as inulin, iohexol, 51CrEDTA, 99mTcDTPA, or 125I-iothalamate. These techniques are time-consuming, labor-intensive, expensive, and require administration of substances that make them incompatible with routine monitoring.
Despite all known disadvantages, serum creatinine concentration has become the most commonly used marker to estimate GFR in clinical practice as in the case of most studies.Citation2 Nevertheless, creatinine-based estimations seem to be low-sensitive markers of renal disease in persons with GFR ≤60 mL/min3; therefore, cystatin C has been recommended as a marker for evaluating renal function. Cystatin C appears to be more sensitive for detecting reduced GFR than either creatinine or estimated GFR.Citation4,5 Although both creatinine and cystatin C are freely filtered in the glomerulus, a major difference is that creatinine is secreted by renal tubules, whereas cystatin C is metabolized by the proximal tubule and only a small fraction appears in the urine.Citation6 Cystatin C has also been shown to be a stronger predictor of adverse outcomes than serum creatinine, so it could be used as a cardiovascular risk marker.Citation7,8
Although serum cystatin C level is used as renal disease progression marker, several equations have been developed to estimate GFR from serum cystatin C.Citation9–15 We compared the results obtained by these equations to test the homogeneity of their results as well as their usefulness in clinical practice.
DESIGN AND METHODS
Seven hundred and twenty-seven Caucasian outpatients were studied. Of these, 439 were male and 288 were female, and their mean age was 60.8 ± 24.1 years. Two hundred and thirty-seven patients were older than 65 years. Kidney function was evaluated by measuring serum cystatin C using a BNII nephelometer (Siemens Diagnostics, Deerfield, IL, USA) through a particle-enhanced immunonephelometric assay (N Latex Cystatin C). The range for the assay was 0.195–7.330 mg/L, with the reference range for young healthy individuals reported as 0.53–0.95 mg/L. The cut off for the highest quartile of serum cystatin distribution was 1.33 mg/L. Mean creatinine value was 1.26 ± 0.75 mg (men 1.31 ± 0.76 vs. women 1.19 ± 0.74, p = 0.032, Student’s t-test).
GFR was estimated from uncalibrated serum creatinine using the abbreviated Modification of Diet in Renal Disease (MDRD-4) equation.Citation16,17 Because Caucasian outpatients were included in this study, they were not included in calculation. Patients were classified according to K/DOQI stages of chronic renal disease following the results of MDRD formulaCitation18: 1.5% were in stage V, 12.1% were in stage IV, 23.3% were in stage III, 18.6% were in stage II, and the remaining patients had GFR higher than 90 mL/min (44.5%). GFR was estimated from serum cystatin C levels using five different equations (all equations are described in ).
Table 1. Equations used to estimate GFR from serum cystatin.
Results are expressed as mean ± 1 SD. All statistical tests were two-sided. p-Values lower than 0.05 were considered significant. Student’s t-test for paired samples was used for continuous variables, and χ2 test was used for categorical variables for comparison between groups. To evaluate concordance among the measures the Pearson Coefficient correlation test was used and receiver operating characteristic (ROC) curves were plotted. Analysis of the test was done using the statistical package PASW 17.0 (Armonk, NY, USA).
RESULTS
shows the differences of estimated GFR among the serum cystatin-derived equations and the classical MDRD-4 equations. The simplest formula rendered the highest estimated GFR and the Hoek equation rendered the lowest GFR, even significantly lower than the MDRD-4 (p < 0.001 Student’s t-test). From the simplest formula (106.0 ± 44.6 mL/min/1.73 m2) to the Hoek equation (81.0 ± 35.7 mL/min/1.73 m2) mean difference was calculated as 25.1 ± 8.7 mL/min (p < 0.001, Student’s t-test).
Table 2. Differences by gender.
No differences by gender were found among the results of different equations. When elderly people (older than 65 years) were separately analyzed, the results were similar but Larrson and Hoek equations had closer results, very near to those estimated using the MDRD-4 formula (see the ).
Table 3. Elderly patients.
Classification of patients with estimated GFR <60 mL/min also showed wide variations from one formula to another. Nevertheless, all cystatin C-derived equations reduced the number of patients diagnosed with chronic renal failure compared with the MDRD-4 formula. The Larsson (n = 11) and Hoek (n = 16) equations had a minor frequency of misclassifications. Any patient diagnosed as not having renal failure by the MDRD-4 formula was reclassified by the other equations, but many patients were excluded from this diagnosis when cystatin C equations were used. shows the differences by frequency of changed diagnosis.
Table 4. Change in the diagnosis of CRF versus MDRD.
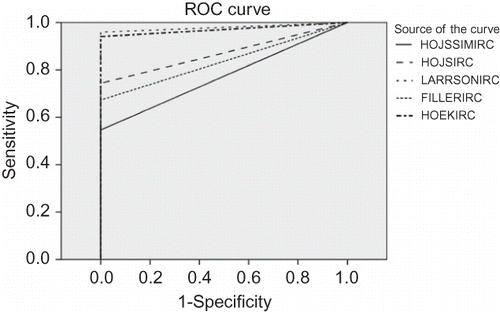
Pearson’s correlation of the MDRD-estimated GFR was highly significant for all the equations: Hojs, 0.999; simplest, 0.999; Hoek, 0.994; Filler, 0.995; and Larsson, 1. ROC curves were plotted for an estimated GFR <60 mL/min using the MDRD-4 equation (). Again, Larsson formula formed a closer relationship with MDRD-4 and the simplest equation had the lowest correlation.
DISCUSSION
Wide differences were observed from one formula to another when GFR was estimated from serum cystatin C. Nevertheless, all cystatin C-derived equations reduced the diagnosis of kidney failure when compared with the usual MDRD-4 equation.
The K/DOQI guidelines recommended estimation of GFR by using prediction equations based on serum creatinine determinations.Citation4 The abbreviated MDRDCitation19 equation was advocated in these guidelines because it correlated well with GFR measured from iothalamate clearance.Citation16 It also performed as a more complicated MDRD equation that required serum urea nitrogen and albumin determinations.Citation17 In fact it is currently the most used equation all over the world. Current guidelines and recommendations for patients with chronic kidney disease (CKD) also call for reducing the dosage of drugs excreted by the kidney, avoiding contrast media for imaging procedures and phosphate-based enemas in preparation for colonoscopy, and setting lower targets for cardiovascular risk factors in patients with decreased GFR.Citation20–22 GFRs falsely estimated as low could therefore lead to insufficient drug dosing, withholding of important diagnostic tests, and overaggressive cardiovascular risk factor reduction in patients without CKD. Therefore, the effect of different estimates of GFRs on clinical decision making should be cautiously evaluated. Our results suggest that MDRD-4 underestimated the GFR, so it should be used cautiously. This problem has been previously reported, and recently, a new formula, the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration),Citation23 has been proposed to improve the estimation of GFR in patients without CKD.
The most common measure used to assess overall kidney function is the plasmatic creatinine concentration. Interpretation of this index is complicated, as it is inversely proportional to the GFR and varies between individuals based on differences in age, sex, and muscle mass. Furthermore, creatinine-based measures seem to be low-sensitive markers of renal disease in persons with GFR ≤60 mL/min/1.73 m3. Cystatin C has been used as an alternative to evaluate renal function. Cystatin C has also been shown to be a stronger predictor of adverse outcomes than serum creatinine, so that it could be a cardiovascular risk marker.Citation7,8
Differences in renal tubular handling of creatinine and cystatin C may have affected their different ability to predict GFR. Creatinine is secreted by tubular cells, increasing with decreasing renal function,Citation24 whereas at least 99% of filtered cystatin C is reabsorbed and degraded by tubular cells.Citation25 Furthermore, both cystatin C and creatinine levels are influenced by changes in thyroid function.Citation26,27 Cystatin C levels are decreased in the hypothyroid state and increased in the hyperthyroid state compared with the euthyroid state, whereas the opposite prevails for creatinine. Large doses of glucocorticoids increase cystatin C levels in contrast to creatinine levels.Citation27
Apart from distinctly different sources of errors between creatinine and cystatin C in estimating GFR, there may still be a number of unrevealed factors affecting the analyses. The marker used to measure GFR may have had a strong influence in the results. Filler and LepageCitation13 validated the (99m)Tc-DTPA single-injection technique to estimate GFR from cystatin C by using nuclear medicine GFR clearance studies. Hojs et al.Citation9 also used a nuclear medicine technique but with a different marker, (51) Cr-EDTA. Both equations rendered close results for estimated GFR in our sample. In fact, these isotopic methods rendered similar results when compared.Citation28 In the Larsson study plasma creatinine and cystatin C were compared with iohexol clearance and two equations were described accordingly in a way that cystatin was calibrated (Dade Behring or DakoZytomation; we selected the first because it was the method used in our laboratory).Citation14 GFR was determined with [125I]iothalamate in the study of Hoek et al.Citation10 This was the same method used to create the MDRD-4 formula, which explains why these two equations (MDRD-4 and Hoek) gave the closest results for the estimation of GFR. It could be concluded that every formula agreed with the method directly used to measure GFR and, most likely, this is the reason for the differences detected.
Sample size and selection criteria could also have some influence. Hoek et al. used plasma samples obtained from 93 consecutive patients (from 11 to 73 years old) with several kinds of CKD and from 30 patients with type 2 diabetes mellitus. Hojs studied 764 adult (older than 18 years) patients with CKD. Filler et al. selected 536 children with various renal pathologies. Larrson et al.Citation29 recruited 94 consecutive patients (ranged 32–94 years old) with iohexol-calculated GFR <30 mL/min/1.73 m2. The etiology of the GFR reduction was not recorded; the homogeneity of samples in relationship to the origin of CKD is unknown. In this regard, most patients in our sample were not CKD patients. On the contrary, big differences could be found in the age of recruited patients, from children to very old people. Moreover, the sample included a small group of advanced stage CKD patients (stages IV and V); these differences could be lesser in this group of population as in the comparison of MDRD and CKD-EPI formulations.Citation23
Recent reports indicate that the arithmeticCitation30,31 or geometric meanCitation12,32 of plasma creatinine and cystatin C-based GFR equations or deriving GFR equations including both plasma creatinine and cystatin CCitation27–33 improves estimated GFR compared with using these parameters separately. Nevertheless, this improvement is small but it could still be valuable and this could be an alternative for getting an assured result while estimating GFR when very big differences are found between both estimations.
Wide differences were found among the equations studied and no recommendation could be made about the best equation to be used for estimating GFR from cystatin C. Higher GFR could be expected than when MDRD-4 is used to estimate this parameter, although Hoek and Larson equations rendered closer values when compared with the other cystatin-derived equations. Nephrologists should be aware of this problem when testing renal function using cystatin C.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Donadio C, Lucchesi A, Ardini M. Cystatin C, b2 microglobulin, and retinol binding proteins as indicators of glomerular filtration rate: Comparison with plasma creatinine. J Pharm Biom Anal. 2001;24:835–842.
- Robles NR. Do we need glomerular filtration rate calculation? Intl J Clin Pract. 2007;67:1611–1613.
- Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937.
- Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83.
- Newman DJ, Thakkar H, Edwards RG, . Serum cystatin C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–318.
- Laterza OF, Price CP, Scott MG. Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707.
- Shlipak MG, Sarnak MJ, Katz R, . Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060.
- Shlipak MG, Sarnak MJ, Katz R, . Cystatin-C and mortality in elderly persons with heart failure. J Am Coll Cardiol. 2005;45:268–271.
- Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L. Kidney function estimating equations in patients with chronic kidney disease. Int J Clin Pract. 2011;65:458–464.
- Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–2031.
- Risch L, Huber AR. Assessing glomerular filtration rate in renal transplant recipients by estimates derived from serum measurements of creatinine and cystatin C. Clin Chim Acta. 2005;356:204–211.
- Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69:399–405.
- Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981–985.
- Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64:25–30.
- Grubb A, Nyman U, Bjork J, . Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem. 2005;51:1420–1431.
- Manjunath G, Sarnak MJ, Levey AS. Prediction equations to estimate glomerular filtration rate: An update. Curr Opin Nephrol Hypertens. 2001;10:785–792.
- Levey A, Bosch J, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis 2002;39(Suppl.1):S1–S246.
- Levey AS, Greene T, Kusek J, Beck G. A simplified equation to predict glomerular filtration rate from serum creatinine (abstract). J Am Soc Nephrol. 2000;11:155A.
- Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107.
- Seliger SL, Zhan M, Hsu VD, Walker LD, Fink JC. Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol. 2008;19:2414–2419.
- Mancia G, De Backer G, Dominiczak A, . Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187.
- Levey AS, Stevens LA, Schmid CH, . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–612.
- Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem. 1992;38:1933–1953.
- Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR history, indications, and future research. Clin Biochem. 2005;38:1–8.
- Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–1947.
- Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47:2055–2059.
- Fleming JS, Wilkinson J, Oliver RM, Ackery DM, Blake GM, Waller DG. Comparison of radionuclide estimation of glomerular filtration rate using technetium 99m diethylenetriaminepentaacetic acid and chromium 51 ethylenediaminetetraacetic acid. Eur J Nucl Med. 1991;18:391–395.
- Jonsson AS, Flodin M, Hansson LO, Larrson A. Estimated glomerular filtration rate (eGFRCystC) from serum cystatin C shows strong agreement with iohexol clearance in patients with low GFR. Scand J Clin Lab Invest. 2007;67:801–809.
- Tidman M, Sjostrom P, Jones I. A Comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant. 2008;23:154–160.
- Stevens LA, Coresh J, Schmid CH, . Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406.
- Ma YC, Zuo L, Chen JH, . Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int. 2007;72:1535–1542.
- Bouvet Y, Bouissou F, Coulais Y, . GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol. 2006;21:1299–1306.