Abstract
Cardiovascular complications are encountered frequently in end-stage renal disease (ESRD) patients. The study was designed as a prospective cohort study and a total of 105 dialysis patients, 77 hemodialysis and 28 peritoneal dialysis patients, were investigated. All patients had undergone M-Mode Doppler echocardiography every 6 months by which their systolic pulmonary arterial pressures (sPAPs) and left ventricular mass indices (LVMIs) were recorded. Thirty-nine (37.1%) patients had pulmonary hypertension (PHT), that is, a mean sPAP of more than 35 mmHg. The frequency of PHT was higher in peritoneal dialysis patients but the difference was insignificant (p = 0.08). However, the frequency of left ventricular hypertrophy (LVH) was found to be significantly higher in peritoneal dialysis patients than in hemodialysis patients (p = 0.001). When patients with and without PHT were compared, the duration of dialysis (p = 0.02), hemoglobin (p = 0.01), HbA1c (p = 0.03), and serum albumin levels (p = 0.003) were found to be significantly higher in patients with PHT than those without PHT. In conclusion, although nonsignificant, we found a higher prevalence of PHT in peritoneal dialysis patients when compared with hemodialysis patients. This might be due to the significantly higher prevalence of LVH, hence hypervolemia, in peritoneal dialysis patients. The prevention and treatment of PHT in dialysis patients is very important for the improvement of survival in these patients. Hence, the increased prevalence of PHT in ESRD patients necessitates understanding the multiple and interacting factors, such as LVH, serum albumin and hemoglobin levels, and control of diabetes, that might contribute to this pathology in these patients.
INTRODUCTION
Pulmonary hypertension (PHT) is a progressive, fatal pulmonary vascular disease that accompanies many conditions, including anemia, fluid overload, left ventricular diastolic dysfunction, and hormonal and metabolic disorders, which all occur in renal failure.Citation1 Cardiovascular complications are common in end-stage renal disease (ESRD) patients.Citation2 PHT is one of those reported, but prevalence and mechanisms have not been widely studied.
Pathogenic mechanisms suggested for PHT in dialysis patients are endothelial dysfunction with reduced nitric oxide levels and alterations in vasoconstrictive and vasodilatory substances leading to an increased pulmonary vascular resistance.Citation3,4 Vascular calcification is also a possible factor.Citation5
Creation of arteriovenous fistulas (AVFs) in hemodialysis patients is another possible mechanism for the development of PHT indicating the possibility that excessive pulmonary blood flow is involved in the pathogenesis of the disease.Citation6,7 Yet, the relationship between AVF blood flow and pulmonary hemodynamics is not very well known. It is suggested that endothelial dysfunction of pulmonary vessels cannot compensate for the AVF-mediated increased cardiac output due to endothelial dysfunction exacerbating PHT.Citation3
The aim of this study was to investigate the prevalence of PHT in dialysis patients, the differences between two dialysis modalities as hemodialysis versus peritoneal dialysis, and possible contributing factors to PHT in ESRD patients.
MATERIALS AND METHODS
The study was designed as a prospective cohort study. ESRD patients on dialysis at least for 3 months at a tertiary referral hospital were recruited into the study. The patients with chronic obstructive pulmonary disease, primary PHT, pulmonary thromboembolic disease, connective tissue disorders with pulmonary involvement, congenital heart diseases and valvular diseases, and the peritoneal dialysis patients with AVFs were excluded from the study. A cohort of 118 dialysis patients was included in the study and were prospectively evaluated for 24 months; 13 of the 118 patients recruited were lost to follow-up either due to switches in the treatment type of ESRD or due to death.
Hence, a total of 105 dialysis patients were investigated all through the study period. Of these patients, 77 (73.3%) were on hemodialysis while 28 (26.7%) were on peritoneal dialysis treatments. Seventy-two hemodialysis patients (93.5%) were receiving treatment via AVFs, while five (6.5%) were receiving treatment via permanent intravascular catheters.
Age and sex of the patients and the presence of diagnosis of diabetes mellitus in the patients were recorded. All patients had undergone M-Mode Doppler echocardiography (echo) every 6 months by which their systolic pulmonary arterial pressures (sPAPs) and left ventricular mass indices (LVMIs) were recorded. PHT was defined as sPAP higher than 35 mmHg at rest, and it was calculated by using the modified Bernoulli equation.Citation8 Left ventricular hypertrophy (LVH) was defined as LVMI higher than 116.0 g/m2 for men and 104.0 g/m2 for women on echo.Citation9 LVMI was calculated using the following equation: LVMI = 0.8{1.04[(LVIDD + PWTD + IVSTD)3 – IVSTD3]} + 0.6 g, where LVIDD was left ventricular internal diameter in diastole, PWTD was posterior wall thickness in diastole, and IVSTD was interventricular septum thickness in diastole.Citation9 For the hemodialysis patients, echoes were performed on the following day of their dialysis treatment. All of the 72 (93.5%) hemodialysis patients with native AVFs also had undergone Doppler ultrasonography (DUSG) of their AVFs every 6 months and the anatomical location (brachial or radial) and flow rate (mL/s) of these AVFs were recorded. Laboratory investigations done for each patient included levels of mean hemoglobin, HbA1c as the marker of blood glucose control in diabetic patients, serum albumin, calcium, inorganic phosphorus (iP), calcium–phosphorous product, inactive parathormone (iPTH), and serum cholesterol.
Differences between groups were compared with Student’s t-test for parametric continuous variables, Mann–Whitney U-test for nonparametric continuous variables, and χ2-test for categorical variables. Pearson correlation coefficient was used to test the relationship between sPAP and AVF flow. A p-value of <0.05 was used as the threshold of statistical significance. All analyses were performed using SPSS 13.0 (IBM Software, New York, NY, USA).
RESULTS
The mean age of this patient population (n = 105) was 61.7 years and 51% were male. Thirty-nine (37%) of all patients were diabetic; 26 of whom were on hemodialysis (34% of hemodialysis patients), while 13 on peritoneal dialysis (46% of peritoneal dialysis patients). The age, sex, and diabetes mellitus distribution were not significantly different between the two types of dialysis treatment ().
Table 1. Demographic characteristics of the patients.
The mean sPAP of all 105 dialysis patients was 28.1 mmHg. Although the mean sPAP was found to be higher in peritoneal dialysis patients (28.7 mmHg), the difference was not significant when compared with that in hemodialysis patients (27.6 mmHg) (p = 0.07). The mean sPAP of diabetic patients regardless of dialysis modality was 26.7 mmHg and higher than nondiabetics (24.1 mmHg) (p = 0.01). Among the 72 hemodialysis patients with AVFs who had undergone Doppler USG, 40 (56%) had brachial fistulas while 32 (44%) had radial fistulas. The mean sPAP was 24.9 mmHg in patients with brachial fistulas and 24.6 mmHg in patients with radial fistulas (p = 0.25) ().
Table 2. The mean sPAP in different patient groups.
Thirty-nine (37.1%) patients had PHT (mean sPAP > 35 mmHg). The frequency of PHT was not significantly different between hemodialysis (33.8%) and peritoneal dialysis patients (35.7%) (p = 0.08). However, the frequency of LVH was found to be significantly higher in peritoneal dialysis patients than in hemodialysis patients (p = 0.001) ().
Table 3. Echocardiographic findings of the patients.
Doppler USG of hemodialysis patients with AVFs revealed a mean fistula flow of 813.75 mL/s for brachial fistulas and 767.19 mL/s for radial fistulas (p = 0.15). Same as the mean fistula flow and levels of sPAP, the frequency of PHT was also higher in patients with brachial fistulas (26.4%) compared to patients with radial fistulas (23.2%), however, again the difference was not significant (p = 0.18) (). The mean levels of sPAP were significantly correlated with mean levels of AVF flow regardless of the location of AVF (r = 0.654, p = 0.001) ().
Figure 1. Correlation between sPAP and AVF flow.Notes: AVF, arteriovenous flow; sPAP, systolic pulmonary hypertension.
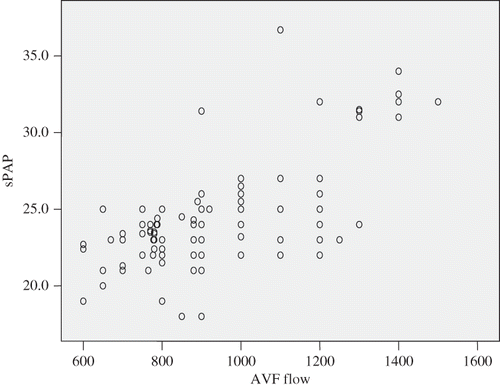
Table 4. Doppler USG findings of the AVFs.
When demographic and laboratory data were compared between patients with and without PHT, some differences were found. The duration of dialysis (p = 0.02), hemoglobin (p = 0.003), and serum albumin (p = 0.001) levels were significantly higher in patients with PHT and HbA1c levels were significantly higher in diabetic patients with PHT (p = 0.002). Age (p = 0.23), serum cholesterol (p = 0.5), Calcium and phosphate (CaXiP) product (p = 0.43), and iPTH levels (p = 0.32) were not different in the two groups ().
Table 5. Comparison between different patient groups.
DISCUSSION
In our study, we found the prevalence of PHT in our dialysis patient population to be 32.4%. In the literature, the prevalence of PHT varies greatly among dialysis centers, in some centers this prevalance increases even upto 50% of the dialysis patients.Citation10–13
Previous studies on PHT were generally on patients from a single dialysis modality, that is, either hemodialysis or peritoneal dialysis. There have been few studies comparing the two dialysis modalities; in one of these studies, Yigla et al.Citation6 found that the prevalence of PHT was high in hemodialysis patients while none was detected in control patients who were peritoneal dialysis patients. In another study done by Tarrass et al.,Citation12 all ESRD patients undergoing transplantation were investigated and they found a significantly higher rate of PHT in hemodialysis patients compared with that of peritoneal dialysis patients. In contrast to these two studies, in our study, we found a higher prevalence of PHT in peritoneal dialysis patients (35.7% vs. 31.2%) although insignificant. Echo findings of peritoneal dialysis patients also showed a significantly higher frequency of LVH, hence a higher state of hypervolemia which may be the reason for the higher prevalence of PHT in these patients.Citation14
We found out the levels of sPAP of diabetic patients to be significantly higher than those of patients without diabetes mellitus (p = 0.02). Endothelial dysfunction is proposed to be a potential mechanism for the development of PHT, expressed by the imbalance between vasodilators and vasoconstrictors of the vascular tone such as nitric oxide and endothelin-1.Citation3,4,10 Hence, the higher prevalence of PHT in diabetic patients may be explained by well-known endothelial dysfunction in these patients.Citation15–17
AVF is a well-studied risk factor for PHT in hemodialysis patients; many studies found that AVF is related and is a potential etiological factor for PHT,Citation3,4,6,7,10 while some others could not show any relationship between PHT and AVF.Citation13,18 Nakhoul et al.,Citation10 in their study showed the regression of PHT and improvement of sPAP to almost normal levels in patients after AVF closure, following renal transplantation. The positive correlation between sPAP and mean AVF flow rate shown by our study was also in concordance with the findings in the literatureCitation7,10 (). Mean flow rates (p = 0.15) and mean sPAPs (p = 0.25) of brachial AVFs were higher than the ones of radial AVFs but the differences were insignificant.
In our study, in contrast to dialysis duration (p = 0.02), age (p = 0.5) was not found to be significantly different in patients with PHT when compared with those without emphasizing the increased risk of PHT in patients on dialysis regardless of aging.Citation12–20
On comparing the patients with and without PHT, levels of hemoglobin (p = 0.01), HbA1c in diabetics (p = 0.03), and serum albumin (p = 0.003) were found to be significantly higher in patients with PHT, while CaXiP product, iPTH, and total cholesterol levels were not found to be significantly different between the two groups of patients (). We believe that anemia, which can be a factor in the development of LVH and increased cardiac output, may help in the pathogenesis of PHT. In our study, the result we found was in favor of this hypothesis; mean hemoglobin levels of dialysis patients with PHT were significantly lower than those without PHT. However in literature, there are results contradicting ours such that they could not show any relationship between hemoglobin levels and sPAP.Citation12,18 HbA1c levels were also significantly lower in diabetic patients without PHT than the ones with PHT. This finding supported our previous finding of the higher sPAP levels in diabetic patients which probably got worsened with bad control of blood glucose shown by high HbA1c levels. In our study, serum albumin levels were also found to be significantly lower in dialysis patients with PHT than without PHT. This finding was also shown in other studies.Citation14,21 It is well known that serum albumin is a marker of malnutrition and inflammation in dialysis patients.Citation22 Since inflammation can cause endothelial dysfunction, hypoalbuminemia is expected to be related with PHT.Citation23,24 Besides this, hypoalbuminemia might be a result of hypervolemia, a cause of PHT.Citation14 Pulmonary calcification is another proposed mechanism for PHT in ESRD patients; however, studies found conflicting results on thisissue.Citation5,25 Kumbar et al.Citation11 found a positive correlation between CaXiP product, iPTH, and sPAP in peritoneal dialysis patients, while other studies could not show any relationship between these parameters and PHT in ESRD patients.Citation13,18,26,27 We could not show any significant difference with respect to CaXiP product and iPTH on comparing the patients with and without PHT (). Total cholesterol levels were not found to be different in two different groups of dialysis patients; hence, we could not relate hyperlipidemia with PHT. We could not find any information about this in the literature.
In conclusion, the prevention and treatment of PHT in dialysis patients is very important for the improvement of survival in these patients.Citation28 Hence, in order to develop strategies in prevention and treatment, the increased prevalence of PHT in ESRD patients necessitates understanding the multiple and interacting factors, such as LVH, hence hypervolemia, serum albumin, hemoglobin, and control of diabetes, that might contribute to this pathology in these patients. Further studies are needed in order to decide the effect of type of dialysis on PHT.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- McLaughlin VV, Archer SL, Badesch DB, . ACCF/AHA 2009 expert consensus document on pulmonary hypertension. A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619.
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal failure. Am J Kidney Dis. 1998;32(Suppl. 5):112–119.
- Yigla M, Abassi Z, Reisner SA, Nakhoul F. Pulmonary hypertension in hemodialysis patients: An unrecognized threat. Semin Dial. 2006;19:353–357.
- Abassi Z, Nakhoul F, Khankin E, Reisner SA, Yigla M. Pulmonary hypertension in chronic dialysis patients with arteriovenous fistula pathogenesis and therapeutic prospective. Curr Opin Nephrol Hypertens. 2006;15:353–360.
- Buemi M, Senatore M, Gallo GC, . Pulmonary hypertension and erythropoietin. Kidney Blood Press Res. 2007;30:248–252.
- Yigla M, Nakhoul F, Sabag A, . Pulmonary hypertension in patients with end stage renal disease. Chest. 2003;123: 1577–1582.
- Beigi AA, Sadeghi AM, Khosravi AR, Karami M, Masoudpour H. Effects of the arteriovenous fistula on pulmonary artery pressure and cardiac output in patients with chronic renal failure. J Vasc Access. 2009;10:160–166.
- Dabestani A, Mahan G, Gardin JM, . Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol. 1987;59:662–668.
- Devereux RB, Wachtell K, Gerdts E, . Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356.
- Nakhoul F, Yigla M, Gilman R, Reisner SA, Abassi Z. The pathogenesis of pulmonary hypertension in hemodialysis patients via arterio-venous access. Nephrol Dial Transplant. 2005;20:1686–1692.
- Kumbar L, Fein PA, Rafiq MA, Borawski C, Chattopadhyay J, Avram MM. Pulmonary hypertension in peritoneal dialysis patients. Adv Perit Dial. 2007;23:127–131.
- Tarrass F, Benjelloun M, Medkouri G, Hachim K, Benghanem MG, Ramdani B. Doppler echocardiography evaluation of pulmonary hypertension in patients undergoing hemodialysis. Hemodial Int. 2006;10:356–359.
- Bozbas SS, Akcay S, Altin C, . Pulmonary hypertension in patients with end stage renal disease undergoing renal transplantation. Transpl Proc. 2009;41:2753–2756.
- Unal A, Sipahioglu M, Oguz F, . Pulmonary hypertension in peritoneal dialysis patients: Prevalence and risk factors. Perit Dial Int. 2009;29:191–198.
- Mokini Z, Marcovecchio ML, Chiarelli F. Molecular pathology of oxidative stress in diabetic angiopathy: Role of mitochondrial and cellular pathways. Diabetes Res Clin Pract. 2010;87:313–321.
- Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286.
- Toda N, Imamura T, Okamura T. Alteration of nitric oxide-mediated blood flow regulation in diabetes mellitus. Pharmacol Ther. 2010;127:189–209.
- Acarturk G, Albayrak R, Melek M, . The relationship between arteriovenous fistula blood flow rate and pulmonary artery pressure in hemodialysis patients. Int Urol Nephrol. 2008;40:509–513.
- Fabbian F, Cantelli S, Molino C, Pala M, Longhini C, Portaluppi F. Pulmonary hypertension in dialysis patients: A cross-sectional Italian study. Int J Nephrol. 2010;30:283475.
- Havlucu Y, Kursat S, Ekmekci C, . Pulmonary hypertension in patients with chronic renal failure. Respiration. 2007;74(5):503–510.
- Mahdavi-Mazdeh M, Alijavad Mousavi S, Yahyazadeh H, Azadi M, Yoosefnejad H, Ataiipoor Y. Pulmonary hypertension in hemodialysis patients. Saudi J Kidney Dis Transpl. 2008;19:189–193.
- Kaysen GA, Kumar V. Inflammation in ESRD: Causes and potential consequences. J Ren Nutr. 2003;13:158–160.
- Kaysen GA. Effects of inflammation on plasma composition and endothelial structure and function. J Ren Nutr. 2005;15:94–98.
- Aguilera A, Sánchez-Tomero JA, Bajo MA, . Malnutrition-inflammation syndrome is associated with endothelial dysfunction in peritoneal dialysis patients. Adv Perit Dial. 2003;19:240–245.
- Yigla M, Keidar Z, Safadi I, Tov N, Reisner SA, Nakhoul F. Pulmonary calcification in hemodialysis patients: Correlation with pulmonary artery pressure values. Kidney Int. 2004;66:806–810.
- Kooman JP, Leunissen KM. Cardiovascular aspects in renal disease. Curr Opin Nephrol Hypertens. 1993;2:791–797.
- Amin M, Fawzy A, Hamid MA, Elhendy A. Pulmonary hypertension in patients with chronic renal failure: Role of parathyroid hormone and pulmonary artery calcifications. Chest. 2003;124:2093–2097.
- Yigla M, Fruchter O, Aharanson D, . Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009;75:969–975.