Abstract
Although cisplatin is a highly effective antineoplastic agent, nephrotoxicity is its major clinical problem. Recently, it was reported that Spirulina, a blue-green algae, has potent antioxidant properties. The aim of this study was to establish the possible protective role of C-phycocyanin (PC), one of the active ingredients of Spirulina, against cisplatin-induced nephrotoxicity. This study was carried out using human kidney-2 (HK-2) cells and male C57BL6 mice. Cells and mice were divided into four groups; untreated control group, PC-treated control group, cisplatin-treated group, and PC plus cisplatin-treated group. The molecular, functional, and structural parameters were measured. PC significantly attenuated blood urea nitrogen, serum creatinine, renal histological damages, and apoptotic cell death in cisplatin-treated mice. The cisplatin-induced cell death was significantly attenuated in cells pretreated with PC. PC also significantly attenuated the elevation of p-ERK, p-JNK, and p-p38 induced by cisplatin treatment. The expression of Bax, caspase-9, and caspase-3 in cisplatin-treated cells were also decreased by PC treatment. In conclusion, PC ameliorates cisplatin-induced nephrotoxicity and, at least in part, suppression of p-ERK, p-JNK, p-p38, Bax, caspase-9, and caspase-3 may be involved in this mechanism.
INTRODUCTION
Cisplatin is one of the most effective antineoplastic agents. However, its nephrotoxicity is a major clinical problem, which can cause severe renal tubular injury and acute renal failure.Citation1,2 Cisplatin causes tubular injury through various pathways, including hypoxia, free radicals, inflammation, and apoptosis, and interactions may occur among these pathways.Citation3,4 Several studies demonstrated the attribution of mitogen-activated protein kinases (MAPKs) in cisplatin nephrotoxicity by induction of apoptosis. Cisplatin activates p38, ERK, and JNK/SAPK in kidneyCitation5 and the inhibition of these molecules by specific inhibitors attenuates cisplatin nephrotoxicity.Citation6–8 ERK and p38 function as upstream signals stimulating tumor necrosis factor-α (TNF-α) production.Citation9 It is also reported that JNK is associated with TNF-α-induced apoptosis.Citation10 Cisplatin-induced nephrotoxicity was associated with an increase of the pro-apoptotic protein Bax, and decrease of the anti-apoptotic protein Bcl-2.Citation11
C-phycocyanin (PC), a constituent of the blue-green algae Spirulina platensis, contains phycocyanobilin, which is an open-chain tetrapyrrole chromophore covalently attached to the apoprotein.Citation12 PC has been reported to possess marked antioxidant and radical scavenging activity.Citation13 Recently, Khan et al.Citation14 reported PC modulates MAPKs, including ERK and p38. A protective effect against oxalate-mediated renal injury was also demonstrated.Citation15 We evaluated whether PC attenuates cisplatin-induced apoptosis in kidney and investigated the possible mechanisms.
MATERIALS AND METHODS
Animals and Drug Treatment
All the experiments were performed on 12-week-old male C57BL/6 mice weighing 28–30 g (Samtako, Kyoung Gi-Do, Korea). The mice were given a standard laboratory diet and water and were cared under a protocol approved by the Institutional Animal Care and Use Committee of the Chungnam National University Medical School. We divided the mice into four groups: untreated mice (n = 7), PC-treated mice (n = 7), cisplatin-injected mice (n = 12), and PC-treated cisplatin-injected mice (n = 12) groups. Four groups of mice were administered a single intraperitoneal injection of either vehicle or cisplatin (12 mg/kg, Choongwae Pharma Co., Seoul, Korea) between 8:00 am and 10:00 am. As the vehicle, 0.9% NaCl was administered in the same manner. PC (50 mg/kg once; Sigma Chemical Co., St. Louis, MO, USA) was administered intraperitoneally 1 h before injection of cisplatin. The animals were fasted and deprived of water overnight (10–12 h) prior to anesthesia and killed. At 72 h after cisplatin injection, the animals were anesthetized with the intraperitoneal administration of a mixture of ketamine (300 μg/kg, Ketalar (r); Bayer, Leverkusen, Germany) and xylazine (0.1 mL/kg, Rompun (r); Bayer) in solution, and then killed.
Renal Function
For renal function evaluation, we checked blood urea nitrogen (BUN) and serum creatinine using chemistry autoanalyzer, Toshiba 200FR (Toshiba Medical Systems Co., Tokyo, Japan).
Tissue Preparation
Tissue preparation was described previously.Citation16 In brief, left kidney was excised immediately, and cut into three transverse sections. A middle piece of the kidney was fixed in 10% buffered formaldehyde, and then embedded in Paraplast (Sherwood Medical, St. Louis, MO, USA). Other pieces of kidney were snap-frozen in liquid nitrogen and kept in deep freezer at −70°C.
Light Microscopy Examination
Light microscopy examination was described previously.Citation16 In brief, pieces of kidney embedded in Paraplast were cut into 4-μm sections and mounted on glass slides. The sections were then deparaffinized with xylene, stained with periodic acid-Schiff, and examined under a light microscope (400× magnification, Dialux 22; Leitz, Milan, Italy). Tubular injury was evaluated by counting the number of necrotic tubules under light microscopy. Necrotic tubules were defined as ones with areas of tubular epithelial cell necrosis involving >50% of the tubule. Ten consecutive fields were examined under 400× magnification and averaged per slide. The percentage of number of necrotic tubules was evaluated. To minimize observer bias, pathologist scored the kidney sections in a blinded fashion.
Cell Culture and Drug Treatment
Human kidney-2 (HK-2), an immortalized proximal tubule epithelial cell line, was purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured. Briefly, cells were passaged every 3–4 days in 100-mm dishes (Falcon, Bedford, MA, USA) using Dulbecco’s modified Eagle’s medium-F12 (Sigma Chemical Co.) supplemented with 10% fetal bovine serum (Life Technologies Inc., Gaithersburg, MD, USA), insulin–transferrin–sodium selenite media supplement (Sigma Chemical Co.), 100 U/mL penicillin, and 100 mg/mL streptomycin (Sigma Chemical Co.). These cells were incubated in a humidified atmosphere of 5% CO2, 95% air at 37°C for 24 h and subcultured at 70–80% confluence. For experimental use, HK-2 cells were plated onto 60-mm dishes in medium containing 10% fetal bovine serum for 24 h and cells were then switched to Dulbecco’s modified Eagle’s medium-F12 with 2% fetal bovine serum for 16 h. These cells were treated with cisplatin (1 mg/mL; Sigma Chemical Co.) in the presence or absence of PC (1 μM; Sigma Chemical Co.) for 6 h. Control cells received buffer only instead of PC and/or cisplatin. The cells were harvested at the end of the treatment for further analysis.
Cell Viability Assay
For cell survival assay, cells were collected after 24 h incubation with vehicle or cisplatin treatment in the presence or absence of PC and surviving cells were counted with Trypan blue staining. The percentage survival was determined by quantization of the relative viable number of treated cells divided by the viable number of untreated cells. For cytotoxicity assay, we used lactate dehydrogenase (LDH)-based in vitro toxicology assay kit (Sigma Chemical Co.). After 24-h incubation with cisplatin treatment in the presence or absence of PC, the release of LDH was measured in media and cell lysate. The procedure followed the manufacturer’s protocol. The results are expressed as the percentage of total LDH activity, which is calculated as follows: LDH activity in media divided by the sum of LDH activity in media and cell lysate. For cell apoptosis assay, we used fluorochrome inhibitor of caspases (FLICA) kit (Immunochemistry Technologies, LLC, Bloomington, MN, USA). In the process of apoptosis, the caspase becomes activated and the FLICA binds to these activated caspases. HK-2 cells were treated with cisplatin or vehicle with or without PC for 24 h. After 24 h, the cells were stained with FLICA following the manufacturer’s instructions.
Western Blot
Western blot was used to measure pERK, pJNK, pp38, Bax, Bcl-2, and caspase-9 proteins as described previously.Citation16 In brief, kidney sections were homogenized in Protein Extraction solution (PRO-PREP, iNtRON, Sungnam-si, Korea). Forty micrograms of total protein was loaded onto a stacking polyacrylamide gel and resolved on an 8% and 15% polyacrylamide gel with a biotinylated molecular weight standard marker. Then, the samples were wet transferred to a 0.2-μm nitrocellulose membrane (Amersham Pharmacia Biotech, Uppsala, Sweden). The blots were blocked for 1 h with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween-20 (TBST) buffer [20 mM Tris–HCl (pH 7.6), 0.8% NaCl, and 0.05% Tween 20] and incubated overnight at 4°C temperature with a 1:1000 p-ERK, p-JNK, p-p38, Bax, Bcl-2, caspase-9 (Cell Signaling Technology, Beverly, MA, USA), and β-actin antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The blots were incubated with 1:1000 secondary anti-rabbit IgG-HRP-linked antibodies or 1:1000 secondary anti-goat IgG-HRP-linked antibodies (Cell Signaling Technology) for 1 h. The bands were detected using enhanced chemiluminescence (Millipore, Billerica, MA, USA), and exposed to films. The optical density for quantification was obtained using Gel-Pro Analyzer version 3.1 (Media Cybernetics, Silver Spring, MD, USA).
Caspase-3 Activity Assay
Caspase-3 activities were determined using a Chemi-Con caspase colorimetric activity assay kit (Chemi-Con, Temecula, CA, USA) as described previously.Citation17 In brief, incubated cells or chopped tissues were incubated in cell lysis buffer for 10 min. After centrifugation (5 min, 10,000 × g), the supernatant was aliquoted and analyzed immediately, according to the manufacturer’s protocol. Fold increase in caspase-3 activity was determined by comparing optical density readings from PC, cisplatin, and PC with cisplatin groups with those of the untreated control group.
Terminal Deoxynucleotidyl Transferase (TdT)-Mediated dUTP-Biotin Nick End Labeling
Apoptosis was detected by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) method using Apoptosis Detection Kit (S7100-KIT; Chemi-Con) as described previously.Citation16 In brief, the 4-μm-thick paraffin embedded sections were dewaxed. The sections were incubated in 0.3% H2O2 at room temperature to eliminate the endogenous peroxidase activity. Proteinase K [10 μg/mL in 0.1 M Tris, 50 mM EDTA (pH 8)] was applied on the sections, which were incubated for 15 min at room temperature. TdT enzyme was applied to the sections, which were incubated in a humidified chamber for 1 h at 37°C to allow extension of the nick ends of the DNA fragments with digoxigenin-dUTP. Color was developed using 0.05% 3,3’-diaminobenzidine (DAB) with 0.006% H2O2 as substrate. For negative controls, distilled water was used instead of TdT enzyme.
Statistical Analysis
The data are reported as mean ± SD. Multiple comparisons among groups were performed using one-way analysis of variance (ANOVA) with the post hoc Bonferroni test correction (SPSS 11.0 for Windows; SPSS Inc., Chicago, IL, USA). The difference between groups was considered statistically significant at p < 0.05.
RESULTS
In Vivo Experiments
Effect of PC on renal function and histology
BUN and serum creatinine levels were significantly increased in cisplatin-treated mice. In contrast, intraperitoneal injection of PC in cisplatin-treated mice reduced BUN and serum creatinine levels significantly compared with cisplatin-treated mice (p < 0.05, ). At 72 h after cisplatin administration, in mice, histological examination revealed loss of brush border, vacuolization and desquamation of epithelial cells in renal tubular epithelium (A). Renal tubular injury was significantly increased in cisplatin-treated mice. PC significantly attenuated renal tubular injury in cisplatin-treated mice (p < 0.05, B).
Figure 1. Effect of PC on renal function. The levels of serum BUN and creatinine at 72 h after cisplatin injection (C, control cells; PC, PC only-treated mice; CIS, cisplatin-treated cells; Cis + PC, PC with cisplatin-treated mice).
Note: *p < 0.05 versus C, **p < 0.05 versus CIS.
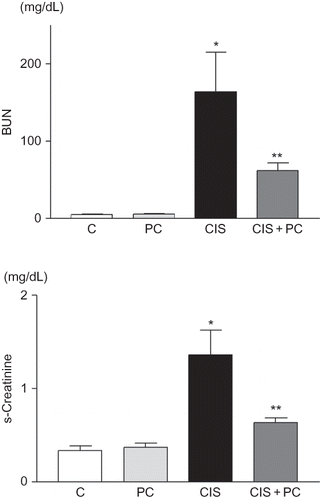
Figure 2. Effects of PC on renal histology (original magnification 100×, 400×). (A) Changes in renal histology 72 h after cisplatin-induced renal injury. The vehicle with cisplatin-treated kidneys showed marked injury, with sloughing of tubular epithelial cells and loss of the brush border. These changes were reduced by PC treatment. (B) Percentage of damaged tubules.
Note: *p < 0.05 versus C, **p < 0.05 versus CIS.

Effect of PC on renal apoptotic cell death
Expression of Bax and Bcl-2. The expression of Bax and Bcl-2 proteins were measured in kidney samples at 72 h after cisplatin treatment. The levels of Bax were increased significantly in kidneys of cisplatin-treated mice compared with the kidneys of the control and PC only-treated mice (p < 0.05, A). Treatment with PC trends toward a decrease in Bax in kidneys of cisplatin-treated mice. Treatment with PC increased Bcl-2 significantly in kidneys of cisplatin-treated mice (p < 0.05, B).
Figure 3. (A, B) Densitometric analysis of Bax and Bcl-2 immunoblots. PC treatment recovered the expression of Bcl-2 protein which was decreased by cisplatin treatment. (C) Representative photomicrographs of Bax and Bcl-2 immunostaining. (D) Quantitative analysis of TUNEL-positive cells. PC treatment reduced TUNEL-positive cells. (E) Colorimetric analysis of caspase-3 activity. PC treatment suppressed caspase-3 activity in kidneys at 72 h after cisplatin treatment.
Note: *p < 0.05 versus C, **p < 0.05 versus CIS.
Caspase-3 activation
Caspase-3 activity increased significantly in kidneys of cisplatin-treated mice, as compared with those of the control and PC only-treated mice (p < 0.05). PC administration reduced caspase-3 activity in kidneys of cisplatin-treated mice (p< 0.05, D).
TUNEL
The number of TUNEL-positive cells was found to increase significantly in kidneys of cisplatin-treated mice compared with the control and PC only-treated mice (p < 0.01). PC administration significantly decreased the number of TUNEL-positive cells (p < 0.05, E).
In Vitro Experiments
Effect of PC on HK-2 cell survival
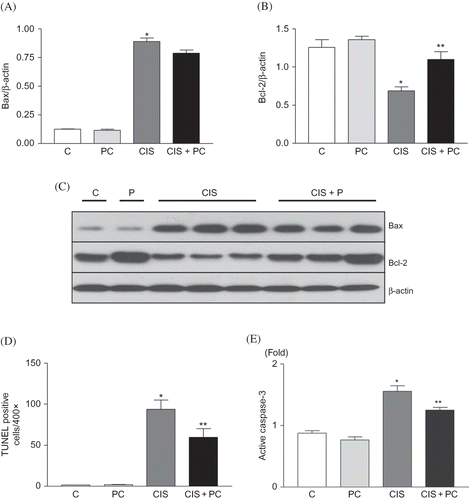
LDH leakage was increased significantly in cisplatin-treated HK-2 cells compared with untreated control and PC only-treated cells. PC treatment reduced LDH leakage in cisplatin-treated HK-2 cells (p < 0.05, A). Trypan blue-positive viable cells were reduced by cisplatin treatment. PC treatment increased trypan blue-positive viable cells in cisplatin-treated HK-2 cells (p < 0.05, B). The number of FLICA-positive cells, apoptotic cells, was increased by cisplatin treatment. PC treatment reduced FLICA-positive cells (p < 0.05, C).
Figure 4. (A) Effect of PC on cytotoxicity and viability in HK-2 cells. (A) LDH leakage was increased in vehicle with cisplatin-treated HK-2 cells. PC treatment reduced LDH leakage in cisplatin-treated HK-2 cells. (B) Viable cells were reduced by cisplatin treatment. PC treatment increased the survival of cisplatin-treated HK-2 cells. (C) Number of FLICA-positive cells, apoptotic cells. PC treatment reduced FLICA-positive cells. (D) Representative photomicrograph of FLICA staining results (original magnification 400×).
Note: *p < 0.05 versus C, **p < 0.05 versus CIS.
Effect of PC on cisplatin-induced HK-2 cell apoptosis
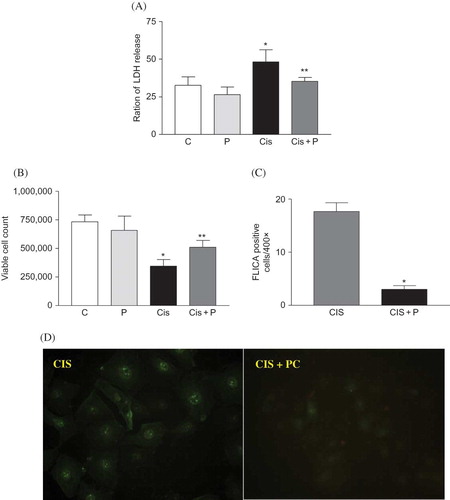
Expression of Bax, Bcl-2, and caspase-9. Bax and Bcl-2 expression were measured in cells at 24 h after cisplatin or vehicle treatment. The levels of Bax were increased significantly in cisplatin-treated cells compared with untreated control and PC only-treated cells (p < 0.05). Treatment with PC reversed Bax in cisplatin-treated cells (p < 0.05, A). Cisplatin treatment reduced the expression of Bcl-2 protein. Treatment with PC increased Bcl-2 significantly in cisplatin-treated cells (p < 0.05, B). Caspase-9 was increased significantly in cisplatin-treated cells compared with untreated control and PC only-treated cells (p < 0.05). Treatment with PC reversed caspase-9 in cisplatin-treated cells (p < 0.05, C).
Figure 5. (A–C) Densitometric analysis of Bax, Bcl-2, and caspase-9 immunoblots. PC treatment decreased the expression of Bax and caspase-9 protein in HK-2 cells at 24 h after cisplatin treatment. PC treatment recovered the expression of Bcl-2 protein which was decreased by cisplatin treatment. (D) Representative photomicrographs of Bax, Bcl-2, and caspase-9 immunostaining. (E) Colorimetric analysis of caspase-3 activity. PC treatment suppressed caspase-3 activity in cisplatin-treated cells at 24 h after cisplatin treatment.
Note: *p < 0.05 versus C, **p < 0.05 versus CIS.
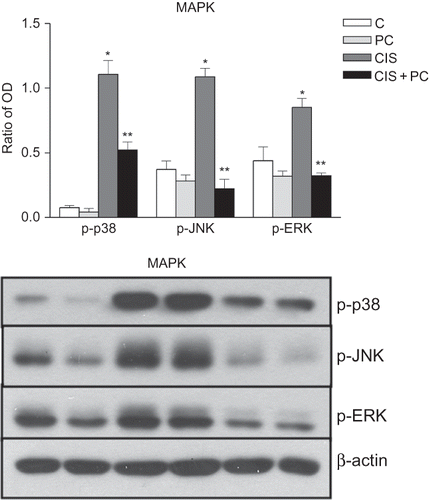
Figure 6. Representative photomicrographs of immunostaining and densitometric analysis for p-p38, p-JNK, and p-ERK. PC treatment decreased phosphorylation of p-p38, p-JNK, and p-ERK in HK-2 cells at 24 h after cisplatin treatment.
Note: *p < 0.05 versus C, **p < 0.05 versus CIS.
Caspase-3 activation
Caspase-3 activity increased significantly in cisplatin-treated cells, as compared with those of the control and PC only-treated cells after 24-h treatment (p < 0.05). PC administration reduced caspase-3 activity in cisplatin-treated cells (p < 0.05, E).
Effect of PC on MAPK of HK-2 cells
Cisplatin treatment trends toward an increased in phosphorylation of p38 and JNK as time goes on (data not shown). PC reduced phosphorylations of p38, ERK, and JNK at 24 h after cisplatin treatment. (p < 0.05, ).
DISCUSSION
In this study, we clearly demonstrated that PC attenuates apoptosis in cisplatin-treated HK-2 cells and renal tissue. The findings of decrease in Bax, increase in Bcl-2, decrease in active caspase-3 and caspase-9, and decrease in TUNEL-positive cells after PC treatment were in accordance with the above results.
Because PC has been reported to possess not only marked antioxidant and radical scavenging activity, but also anti-apoptotic effects,Citation13,18 we suggested that PC is a good candidate for reducing cisplatin-induced nephrotoxicity.
Cisplatin-induced nephrotoxicity can result in histological alterations in renal tubular cells associated with renal dysfunction such as severe reduction in the glomerular filtration and increase in serum creatinine and BUN.Citation19–21 The levels of serum creatinine and BUN are important because they correlate well with the decline and recovery of renal function. In this study, we used them as indices of functional nephrotoxicity.
The in vivo mechanisms of cisplatin nephrotoxicity are complex involving oxidative stress, apoptosis, and inflammation.Citation3,16,22,23 Apoptosis is recently recognized as an important mode in cisplatin nephrotoxicity. Cisplatin induces Fas clustering into cell membrane lipid rafts and induces apoptosis in cancer cells, although association with nephrotoxicity is not clear.Citation24 In addition, cisplatin-induced apoptosis of renal cells was attenuated in caspase 1-deficient mice.Citation25 Bax protein is associated with cisplatin nephrotoxicity. Cisplatin-induced apoptosis was reduced in Bax-deficient mice compared with wild-type mice.Citation26 Cisplatin treatment reduced the expression of Bcl-2 protein in BMK cells. And cisplatin-induced apoptosis was reduced by Bcl-2 transfection in BMK cells.Citation27 Cisplatin activates initiator caspase-9 and executioner caspase-3.Citation28
In our study, PC treatment resulted in reduced apoptosis in cisplatin-treated cells and mice kidneys. In HK-2 cell, the expression of Bax was decreased significantly by PC treatment. However, in mice kidneys, the expression of Bax was not decreased significantly by PC treatment. Moreover, comparing with cisplatin-treated HK-2 cells, cisplatin-treated mice kidneys showed reduced recovery of Bcl-2 expression by PC treatment. These differences may be due to the discrepancy of cell proportion. Mice kidneys contain variable cells, including podocytes, endothelial cells, mesangial cells, proximal tubule cells, and collecting duct cells. However, HK-2 cell is only proximal tubular cell.
It has been known that cisplatin increases phosphorylation of ERK, JNK, and p38 in various tissues,Citation29–31 including kidney.Citation5 It had been demonstrated that p38 MAPK pathway activation was involved in cisplatin-induced nephrotoxicity, and that p38 MAPKs had an important role in the regulation of apoptosis pathways.Citation7,32 Other group proposed that activation of JNK was responsible for cisplatin-induced apoptosis,Citation8 although no significant differences were found in phosphorylated JNK levels between groups with or without antioxidant treatment after the induction of cisplatin nephrotoxicity in other study.Citation9 The role of ERK in cisplatin-induced apoptosis is not clear. Some researchers suggested ERK activation plays an active role in cisplatin-induced apoptosis in HeLa cells. They showed inhibition of ERK activation by PD98059 reduced cisplatin-induced apoptosis in HeLa cells.Citation29 However, Persons et al.Citation31 reported that inhibition of ERK activation by PD98059 enhanced cisplatin-induced cell death in human epithelial adenocarcinoma ovarian cells. In our study, PC treatment resulted in decrease in MAPK activity in cisplatin-induced HK-2 cell injury. It is not clear whether PC acts as a direct MAPK inhibitor. Because PC has potent antioxidant and radical scavenging activity, it may be possible that reactive oxygen species (ROS) or oxidative stress reduced by PC treatment may influence decrease in MAPK activation in cisplatin-induced cell injury. ROS or oxidative stress induced by cisplatin increases activation of p38 or ERK.Citation6,33
In conclusion, the results of this study demonstrate that PC improved the recovery of cisplatin-induced renal injury in renal tissue and HK-2 cell. PC treatment resulted in decrease in p-ERK, p-JNK, p-p38, Bax, caspase-9, and caspase-3; an increase in Bcl-2; and, at least in part, these findings may be involved in this mechanism.
ACKNOWLEDGMENT
This study was financially supported by research fund of Chungnam National University in 2008.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Jones BR, Bhalla RB, Mladek J, . Comparison of methods of evaluating nephrotoxicity of cis-platinum. Clin Pharmacol Ther. 1980;27(4):557–562.
- Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8(5):368–379.
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. Am J Med Sci. 2007;334(2):115–124.
- Engineer MS, Brown NS, Ho DH, Newman RA, Bulger RE. A comparison of the effects of tetraplatin and cisplatin on renal function and gentamicin pharmacology in rats. Toxicology. 1989;59(2):151–162.
- Arany I, Megyesi JK, Kaneto H, Price PM, Safirstein RL. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am J Physiol Renal Physiol. 2004;287(3):F543–F549.
- Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005;67(2):458–466.
- Francescato HD, Costa RS, Da Silva CG, Coimbra TM. Treatment with a p38 MAPK inhibitor attenuates cisplatin nephrotoxicity starting after the beginning of renal damage. Life Sci. 2009;84(17–18):590–597.
- Francescato HD, Costa RS, Junior FB, Coimbra TM. Effect of JNK inhibition on cisplatin-induced renal damage. Nephrol Dial Transplant. 2007;22(8):2138–2148.
- Luo J, Tsuji T, Yasuda H, . The molecular mechanisms of the attenuation of cisplatin-induced acute renal failure by N-acetylcysteine in rats. Nephrol Dial Transplant. 2008;23(7):2198–2205.
- Ding WX, Yin XM. Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury. J Cell Mol Med. 2004;8(4):445–454.
- Tsuruya K, Ninomiya T, Tokumoto M, . Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003;63(1):72–82.
- Lissi EA, Pizarro M, Aspee A, Romay C. Kinetics of phycocyanine bilin groups destruction by peroxyl radicals. Free Radic Biol Med. 2000;28(7):1051–1055.
- Romay C, Armesto J, Remirez D, . Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res. 1998;47(1):36–41.
- Khan M, Varadharaj S, Ganesan LP, . C-phycocyanin protects against ischemia-reperfusion injury of heart through involvement of p38 MAPK and ERK signaling. Am J Physiol Heart Circ Physiol. 2006;290(5):H2136–H2145.
- Farooq SM, Asokan D, Sakthivel R, Kalaiselvi P, Varalakshmi P. Salubrious effect of C-phycocyanin against oxalate-mediated renal cell injury. Clin Chim Acta. 2004;348(1–2):199–205.
- Lee KW, Jeong JY, Lim BJ, . Sildenafil attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Toxicology. 2009;257(3):137–143.
- Choi DE, Jeong JY, Lim BJ, . Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009;297(2):F362–F370.
- Li XL, Xu G, Chen T, . Phycocyanin protects INS-1E pancreatic beta cells against human islet amyloid polypeptide-induced apoptosis through attenuating oxidative stress and modulating JNK and p38 mitogen-activated protein kinase pathways. Int J Biochem Cell Biol. 2009;41(7):1526–1535.
- Evenepoel P. Acute toxic renal failure. Best Pract Res Clin Anaesthesiol. 2004;18(1):37–52.
- Kang DG, Lee AS, Mun YJ, . Butein ameliorates renal concentrating ability in cisplatin-induced acute renal failure in rats. Biol Pharm Bull. 2004;27(3):366–370.
- Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365(9457):417–430.
- Francescato HD, Costa RS, Scavone C, Coimbra TM. Parthenolide reduces cisplatin-induced renal damage. Toxicology. 2007;230(1):64–75.
- Iseri S, Ercan F, Gedik N, Yuksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230(2–3):256–264.
- Dimanche-Boitrel MT, Meurette O, Rebillard A, Lacour S. Role of early plasma membrane events in chemotherapy-induced cell death. Drug Resist Updat. 2005;8(1–2):5–14.
- Faubel S, Ljubanovic D, Reznikov L, . Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int. 2004;66(6):2202–2213.
- Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007;72(1):53–62.
- Jiang M, Wang CY, Huang S, Yang T, Dong Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am J Physiol Renal Physiol. 2009;296(5):F983–F993.
- Kaushal GP, Kaushal V, Hong X, Shah SV. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001;60(5): 1726–1736.
- Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000;275(50):39435–39443.
- Sanchez-Perez I, Murguia JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16(4):533–540.
- Persons DL, Yazlovitskaya EM, Cui W, Pelling JC. Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: Inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clin Cancer Res. 1999;5(5):1007–1014.
- Ramesh G, Kimball SR, Jefferson LS, Reeves WB. Endotoxin and cisplatin synergistically stimulate TNF-alpha production by renal epithelial cells. Am J Physiol Renal Physiol. 2007;292(2):F812–F819.
- Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol. 2005;289(1):F166–F174.
