Abstract
Alpha-lipoic acid (ALA) is a powerful antioxidant, and its effect in ameliorating complications of diabetes mellitus has been widely documented. The aim of this study was to investigate the role of ALA in the disease progression of remnant kidneys (RK). Systolic blood pressure (SBp), hemoglobin, renal function, kidney malondialdehyde (MDA), glutathione (GSH), vitamin E (Vit E) concentrations, p65 nuclear factor (NF)-κB activity, and macrophage infiltration were analyzed in sham and RK rats supplemented with ALA (100 mg/kg body weight, i.p., every other day) or vehicle for 12 weeks. RK rats exhibited increases in SBp, kidney MDA concentration, p65 NF-κB activity, and macrophage infiltration, which were prominent in weeks 4 and 8 and decreased at week 12. RK rats also showed anemia, microalbuminuria, and decreased renal function and kidney GSH and Vit E concentrations. These changes were all attenuated by 8 weeks of ALA treatment compared to RK vehicle group. But the continued ALA treatment after week 8 reversed the beneficial effect on SBp, kidney MDA concentration, p65 NF-κB activity, macrophage infiltration, anemia, microalbuminuria, and renal function, while the beneficial effect was maintained if the treatment was discontinued after week 8. Furthermore, ALA increased albuminuria and kidney MDA concentration in sham animals. In conclusion, ALA administration attenuates oxidative stress, inflammation, and hypertension and delayed the deterioration of kidney function in RK rats with enhanced oxidative stress, while in healthy animals or RK rats with a well-balanced redox state, ALA may act as a pro-oxidant, contributing to renal dysfunction.
INTRODUCTION
Nephron reduction by subtotal nephrectomy in experimental animals or by various diseases in humans triggers a chain of events that lead to glomerulosclerosis, tubulointerstitial injury, proteinuria, and progression to end-stage renal disease. Progressive deterioration of kidney function and structure in the remnant/diseased kidney is mediated by glomerular hypertension and hyperfiltration, as well as oxidative stress and inflammation. Oxidative stress may cause inflammation and fibrosis by direct toxic effects of reactive oxygen species (ROS) and by inducing activation of redox-sensitive proinflammatory transcription factors and signal transduction pathways. Therefore, oxidative stress and its frequent companion, inflammation, play a major role in the pathogenesis of the progression of renal injury as well as in the development of various complications of chronic renal failure, such as hypertension and anemia.Citation1 Oxidative stress in chronic kidney disease (CKD) is caused by a combination of excessive ROS production and antioxidant depletion. Therefore, antioxidants have been considered for prevention of CKD progression by reducing oxidative stress, but available data are limited. Besides, it is also noted that the use of high doses of antioxidant vitamins in humans has been hampered by the general lack of significant demonstrable benefit and the emerging evidence of adverse outcome found in clinical trials.Citation2
Alpha-lipoic acid (ALA) is an endogenous thiol antioxidant, which was originally isolated from the bovine liver in 1950.Citation3 Both ALA and its metabolites possess powerful potential to quench ROS, regenerate glutathione (GSH) and chelating metals, such as iron, copper, mercury, and cadmium, known to mediate free-radical damage in biological systems. In the past decade, there has been a growing body of experimental and clinical evidence that ALA is an useful agent for treating arteriosclerosis, neuron degeneration, joint diseases, peritoneal adhesions, and intestinal ischemia/reperfusion injury,Citation4–8 especially diabetic nephropathy, the complication associated with diabetes mellitus, by attenuating albuminuria, preventing renal insufficiency, glomerular mesangial matrix expansion, and glomerulosclerosis.Citation9–11 Given the critical role of oxidative stress and inflammation in the progression of renal disease, and the potent antioxidant/anti-inflammatory actions of ALA, we hypothesized that long-term administration of this agent might attenuate or delay the deterioration of renal function and structure in rats with renal mass reduction.
MATERIAL AND METHODS
Experimental Protocol
All experiments were performed with male Sprague–Dawley rats (180–200 g) obtained from the Animal Centre, Shanghai Medical College, Fudan University (Shanghai, China). Rats were allowed free access to water and rodent food. All protocols were approved by the Institutional Animal Care Use Committee of Fudan University.
Subtotal nephrectomy was performed in two steps as Tain described previously.Citation12 In brief, two poles of the left kidney were removed first, and then 1 week later, the right kidney was also removed. The sham-operated rats (sham group) underwent the same surgical procedure, except for the resections. All the surgical procedures were carried out under general anesthesia (sodium pentobarbital, 40 mg/kg, i.p.). The rats were then randomly assigned to the following remnant kidney (RK) groups (see ):
Group 1: RK wk 4 (n = 6), treated with vehicle beginning at week 1 every other day until being killed at week 4.
Group 2: RK wk 8 (n = 6), treated with vehicle until being killed at week 8.
Group 3: RK wk 12 (n = 6), treated with vehicle until being killed at week 12.
Group 4: RK + 4 wks ALA (n = 6), RK rats administered ALA until being killed at week 4.
Group 5: RK + 8 wks ALA (n = 6), RK rats administered ALA until being killed at week 8.
Group 6: RK + 12 wks ALA (n = 6), RK rats administered ALA until being killed at week 12.
Group 7: RK + 8 wks ALA′ (n = 6), RK rats administered ALA for the first 8 weeks and then received vehicle instead for the following 4 weeks and killed at week 12.
Group 8: sham wk 12 (n = 4), Sham-operated rats administered vehicle until being killed at week 12.
Group 9: sham + 12 wks ALA (n = 5), Sham-operated rats administered ALA until being killed at week 12.
Blood samples were collected through cardiac puncture when the rats were killed. One piece of kidney tissue was fixed in neutral formalin and then embedded in paraffin. A second piece of the kidney was dissected in ice-cold PBS to remove the medulla and then snap-frozen in liquid nitrogen before being transferred to storage at −80°C until further analysis.
Blood Pressure and Hemoglobin and Blood Biochemistry and 24 h Urine Microalbumin
Systolic blood pressure (SBp) was determined in conscious rats by the tail-cuff method before the rats were killed. Hemoglobin was determined with a hematology analyzer (Sysmex Kx-21; Sysmex, Kobe, Japan). Blood urea nitrogen and serum creatinine (Scr) levels were determined using commercial kits (Urea FS, DiaSys Diagnostic System GmbH, Frankfurt, Germany; CREA plus, Roche, Mannheim, Germany) by an automatic analyzer (Hitachi 7180; Hitachi, Tokyo, Japan). Rats were housed in metabolic cages for overnight collection of 24 h urine before being killed. Urine microalbumin concentration was determined using a Rat Microalbumin ELISA kit according to the manufacturer’s instructions (Kamiya Biomedical Company, Seattle, WA, USA).
Renal Morphologic Analyses
Two-micrometer-thick sections were processed for periodic acid-Schiff staining. The extent of glomerular sclerosis and tubulointerstitial injury was assessed semiquantitatively. Glomerular sclerosis was quantified per glomerulus: grade 0, no sclerosis; grade 1, <25%; grade 2, <50%; grade 3, <75%; and grade 4, ≥75% involved. At least 30 glomeruli were evaluated under ×400 magnification and averaged.Citation14 Tubulointerstitial injury score was obtained based on the morphological changes of the tubules such as dilatation, distortion of tubular basement membranes, and atrophy as follows: grade 0, no morphological deformities; grade 1, <10%; grade 2, <25%; grade 3, <50%; grade 4, <75%; and grade 5, ≥75% involved. More than 20 consecutive fields were examined under ×400 magnification and averaged per slide.Citation14
Parameters of Oxidative Stress and Inflammation
Tissue contents of malondialdehyde (MDA), GSH, and vitamin E (Vit E) in the renal cortical homogenate were measured individually according to the manufacturer’s instructions (Jiancheng Biologic, Nanjin, China). Briefly, MDA was measured based on the spectrophotometric measurement of the color produced during the reaction of thiobarbituric acid with MDA. GSH was measured based on the spectrophotometric measurement of the color produced during the reaction of DTNB and thiol compounds. Vit E was measured based on the spectrophotometric measurement of the color produced during the reaction of divalent iron and o-phenanthrolene. Renal cortex p65 nuclear factor (NF)-κB activity was quantified by ELISA using commercial kits according to the manufacturer’s instructions (Active Motif, Carlsbad, CA, USA).
Immunohistology was used to identify infiltration of macrophages (ED1-positive cells). Details of this technique in our laboratory have been published previously.Citation15 Positive cells were evaluated in tubulointerstitial areas of the cortex (positive cells/×200 visual field).
RESULTS
General Data and Renal Function
Data obtained at the observation endpoint of week 12 after renal ablation are summarized in . Compared with control RK rats, hypertension resulting from renal ablation was ameliorated by both 8 weeks of ALA treatment (RK + 8 wks ALA′ group) and 12 weeks of ALA treatment (RK + 12 wks ALA group). Decreased body weight, increased Scr, and 24 h urine microalbumin, and decreased hemoglobin were seen in rats with renal ablation and were all significantly improved by 8 weeks of ALA treatment; but 12 weeks of treatment had no effect on body weight, Scr, or 24 h urine microalbumin, and even exacerbated anemia compared with RK group. Furthermore, 12 weeks of ALA supplementation increased microalbumin excretion (5.3-fold) in sham animals compared to sham + vehicle, but there was no difference in body weight, SBp, Scr, or hemoglobin between the two groups.
Table 1. Data at the end of the experiment (12 weeks).
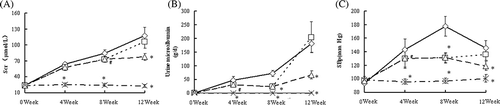
The longitudinal measurements of SBp, Scr, and proteinuria are shown in . Control RK rats exhibited increased SBp that peeked at week 8 and then decreased a little following renal mass reduction. ALA treatment decreased SBp in the RK rats as early as week 4, and this effect continued even after the cessation of ALA treatment at week 8. In contrast, the prolonged ALA treatment after week 8 worsened SBp. Similarly, the protective effect of ALA on Scr and proteinuria on RK was sustained after the cessation of treatment at week 8, and the prolonged ALA treatment even reversed this protective effect.
Figure 2. SBp, Scr, and 24 h urinary protein excretion values during the study in the RK + vehicle group (◊), RK + 12 weeks ALA treatment group (□), RK + 8 weeks ALA treatment group (∆) and sham + vehicle group (×) group. 0 indicates the time when surgery (RK or sham operations) was done.
Notes: Data are mean ±SE. *p < 0.01 versus all other treatment groups, #p < 0.05 versus RK.
Renal Histology
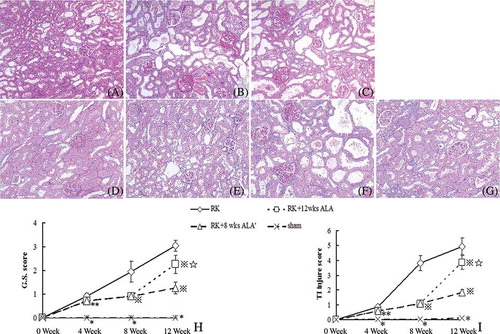
As shown in , control RK rats developed significant and progressive glomerulosclerosis and tubulointerstitial damage. ALA administration significantly reduced the severity of glomerulosclerosis and tubulointerstitial damage at week 8, and this effect did continue after the cessation of treatment at this time; in contrast, the prolonged ALA treatment after week 8 worsened renal tissue damage. Treatment of sham rats with 12 weeks of ALA had no influence on histological changes compared with sham + vehicle (see supplemental Figure 1 available online at http://informahealthcare.com/doi/suppl/10.3109/0886022X.2012.691012).
Figure 3. Effects of ALA on renal histology. Representative pictures of PAS staining are shown (magnification ×100). (A) RK wk 4 group; (B) RK wk 8 group; (C) RK wk 12 group; (D) RK + 4 wks ALA group; (E) RK + 8 wks ALA group; (F) RK + 12 wks ALA group; (G) RK + 8 wks ALA′ group. ALA treatment improved glomerulosclerosis and tubulointerstitial fibrosis before week 8 and this effect continued after the treatment was discontinued after week 8. Continued treatment after week 8 exacerbated renal tissue damage. Semiquantitative scoring of glomerular sclerosis and tubulointerstitial injury was summarized in H–I.
Notes: *p < 0.01 versus the rest, #p < 0.05 versus RK, ⋇p < 0.01 versus RK, *p < 0.01 versus RK + 8 wks ALA′.
Oxidative Stress
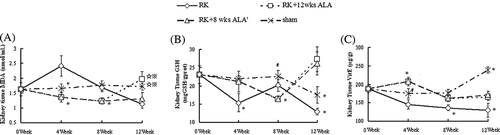
Oxidative stress was evaluated by determining the contents of MDA, GSH, and Vit E in the RK. As shown in , the renal MDA level increased after renal mass reduction and this upregulation was completely prevented by ALA until week 8. After that, the increased MDA level in control RK rats dropped and was even lower than sham rats at week 12; the cessation of ALA treatment at this stage maintained the beneficial effect on MDA, whereas the prolonged treatment reversed this effect and the MDA level at week 12 sharply increased, which was much higher than untreated RK rats at this time point (p < 0.01). Renal GSH and Vit E levels were decreased after renal mass reduction, and 8 weeks later, the levels were even lower. ALA treatment effectively increased Vit E levels in the whole duration of observation, and cessation or continuation of the treatment made no difference on Vit E. Compared with control rats, 4 weeks of ALA treatment effectively increased GSH levels, but 8 weeks of ALA treatment failed to do so. However, following the cessation of ALA, kidney GSH increased beyond the pre-surgery level, and continued ALA treatment after week 8 also increased GSH level to a similar degree. In addition, kidney MDA remained stable in sham rats during the observation period whereas decreased GSH and increased Vit E were seen at week 12 in sham kidneys. Twelve weeks of ALA increased MDA (1.7-fold) in sham kidneys compared to sham + vehicle (see ), and no significant differences were found in GSH or Vit E in the two groups.
Figure 4. Kidney contents of MDA, GSH, and Vit E during the study in the RK + vehicle group (◊), RK + 12 weeks ALA treatment group (□), RK + 8 weeks ALA treatment group (∆) and sham + vehicle group (×) group. 0 indicates the time when surgery (RK or sham operations) was done. Data are mean ± SE.
Notes: *p < 0.01 versus the rest, #p < 0.05 versus RK, ⋇p < 0.01 versus RK, *p < 0.01 versus RK + 8 wks ALA′.
Inflammation Reaction
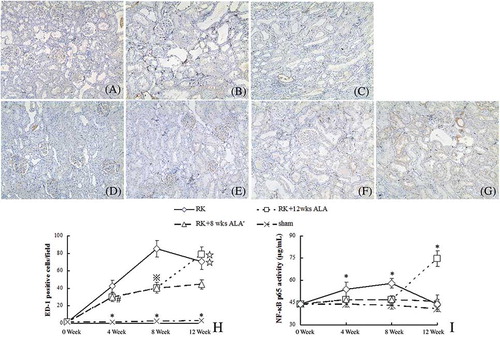
After renal mass reduction, macrophage infiltration was evident in the tubulointerstitium area, especially in week 8 (see A–H); likewise, NF-κB/DNA binding activity in RK rats was increased at that time point (see ). The administration of ALA significantly reduced tubulointerstitial macrophage infiltration and NF-κB/DNA binding activity, and this effect persisted after the cessation of the treatment at week 8. On the contrary, the continued treatment after week 8 aggravated macrophage infiltration and intensified NF-κB/DNA binding activity.
Figure 5. Macrophage (ED-1 positive cells) infiltration (immunohistology, A–H) and renal cortex p65 nuclear factor (NF)-κB activity (I). Immunohistological magnification ×200. (A) RK wk 4 group; (B) RK wk 8 group; (C) RK wk 12 group; (D) RK + 4 wks ALA group; (E) RK + 8 wks ALA group; (F) RK + 12 wks ALA group; (G) RK + 8 wks ALA’ group; (H) Semiquantitative score of ED-1 positive cells infiltration.
Notes: *p < 0.01 versus the rest, #p < 0.05 versus RK, ⋇p < 0.01 versus RK, *p < 0.01 versus RK + 8 wks ALA′.
DISCUSSION
The salient findings of this study were (1) oxidative stress and inflammation in RKs were prominent in the progression of RK rats except the final stage examined; (2) the administration of 100 mg/kg ALA for the first 8 weeks prevented the increase in microalbuminuria, hypertension, renal dysfunction, anemia, and development of glomerulosclerosis and tubulointerstitial fibrosis associated with renal mass reduction by suppressing the oxidative stress and inflammation in RK rats; (3) the supplementation of ALA at the final stage of RK model examined exacerbated renal dysfunction by increasing oxidative stress and inflammation; (4) the supplementation of the same dose of ALA slightly increased oxidative stress without influencing renal function in sham kidney.
The RK model is widely considered to be a classic model of CKD. Abundant evidence has accumulated indicating that CKD causes oxidative stressCitation15–17 and oxidative stress accelerates the progression of renal injury directly by inducing cytotoxicity and indirectly by promoting inflammation.Citation1,16–18 Oxidative stress is a known feature of CKD, and its presence is evidenced by the reported elevation of lipid peroxidation products, MDA, and depressed antioxidant capacity.Citation17,19,20 However, our study demonstrates that after renal mass reduction, MDA level in kidney was highest at week 4 when tubular hypertrophy reached the greatest extent, but then decreased and was even lower than the sham rats after week 8, accompanied by the majority of tubular atrophy and fibrosis with less macrophage infiltration. As to antioxidant, GSH and Vit E remained at low levels in the progression of RK. Thus, oxidative stress resulting from the imbalance between pro- and antioxidants exists in the progression of CKD and returns to normal at the final stage in our RK model.
There has been a significant rise in the number of publications confirming beneficial effects of ALA in the treatment of diabetes and its complications including diabetic retinopathy and diabetic polyneuropathy, while the efficiency in treating kidney disease has occasionally been reported. Bhatti demonstrated that streptozotocin-induced diabetic rats fed lipoic acid at 30 mg/kg body weight for 12 weeks had lower albuminuria and glomerulosclerosis indexCitation11; also the protective effects of ALA on vancomycin-induced nephrotoxicity, cisplatin-induced nephrotoxicity, and kidney ischemia/reperfusion injury have been reported.Citation21–27 But there are no data to suggest the potential effect of ALA on RK, the typical CKD model.
Other investigators have used antioxidant therapy in RK rats. Administration of the superoxide dismutase mimetic tempol for 2 weeks failed to alter renal function in 5/6 nephrectomized rats.Citation28,29 The traditional antioxidant, Vit E, was found to cause significant reduction of glomerulosclerosis but no change in creatinine clearance or proteinuria with supplementation for 15 weeks in 5/6 nephrectomized rats.Citation12 Similar findings were reported by Van den Branden et al. and Hahn et al.Citation30,31 Our results showed not only a marked structural improvement but also an impressive preservation of renal function and reduction of proteinuria with ALA administration in the progression of this model. Furthermore, these benefits can be maintained after the cessation of treatment. Several reasons may explain the superior benefits obtained with ALA. First, ALA and its reduced dithiol form, dihydrolipoic acid (DHLA), are powerful antioxidants that can scavenge hydroxyl radicals, singlet oxygen, hydrogen peroxide, hypochlorous acid, peroxynitrite, and nitric oxide, and the category is much broader than those of Vit E.Citation5 Second, ALA/DHLA redox couple can regenerate exogenous and endogenous antioxidants such as Vit C and E, and GSH.Citation5 Finally, the couple exerts additional antioxidant actions through the chelation of copper, iron, and other transitional metals.Citation5
ALA administration resulted in significant reduction of the lipid peroxidation product MDA in the RK tissues of the RK + 8 wks ALA group. This observation points to the effectiveness of ALA in ameliorating kidney oxidative stress. In addition to its potent antioxidant properties, ALA serves as a potent anti-inflammatory agent. For instance, ALA prevents translocation and DNA binding of transcription factor NF-κB and, thereby, suppresses subsequent production and release of proinflammatory cytokines, chemokines, and adhesion molecules.Citation32,33 This possibility is supported by significant reduction of cells expressing p65, the active DNA-binding subunit of NF-κB as well as the interstitial inflammatory infiltrate in the RK of the ALA-treated rats (see ).
An unexpected finding in this study was that the continued supplementations with ALA after week 8 in RK rats, at doses that prevent the decline in renal function and pathology before week 8, are detrimental. In RK rats after week 8, treatment with ALA is associated with a decline in creatine clearance, increase in albuminuria, glomerulosclerosis, tubulointerstitial fibrosis, and increases in renal lipid peroxidation product MDA, NF-κB p65 activity as well as the interstitial inflammatory infiltrate. Besides, supplementation with ALA in sham rats also increases albuminuria excretion and renal MDA production. These findings suggest that under certain conditions, the antioxidant supplements may exhibit pro-oxidant properties and even worsen renal damage. One possible explanation of this paradox is that the ultimate effect of antioxidant therapy depends on the redox state of targeted animals/patients, and on the timing of such treatment. Only individuals with impaired antioxidant defense, or those faced with conditions of oxidative stress, may benefit from antioxidant therapy.Citation34 We have noticed that the oxidative stress in RK rats after week 8, which was the end stage of RK, returned to normal, and the redox state in sham rats was also balanced. Under these conditions, giving antioxidant has deleterious effects. Thus, our results suggest that antioxidant administration may not be risk-free in all circumstances. A more complete understanding of redox events and oxidant–antioxidant networks at the cellular level is required before giving reasonable recommendations regarding antioxidant therapy.
The importance of oxidative stress in the development and maintenance of hypertension has been recognized for some time,Citation35 and a variety of antioxidant strategies improve hypertension with or without CKD.Citation17,18,35 It has also been reported that ALA treatment can improve hypertension.Citation36,37 Our results show that ALA supplementation can clearly lower the systemic blood pressure of RK rats, accompanied by the improvement of oxidative stress in the kidney. Besides, unlike other antioxidants,Citation35 the cessation of ALA after RK week 8 cannot reverse the lowered blood pressure, whereas the prolonged ALA treatment aggravated the hypertension, which is in accordance with the different redox state in these circumstances.
Based on the findings of our study, we conclude that ALA administration attenuated oxidative stress, inflammation, and hypertension and attenuated the deterioration of the RK function and structure in RK rats with oxidative stress. But when used in individuals with well-balanced redox state, it may have potential harmful effects. Therefore, antioxidant prescription should be carefully considered in selected CKD states.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Funds of China (81000307) and the Project of Technology Committee in Shanghai of China (10JC1402900). The excellent assistance with animal experiments of Dr. Chunlai Lu is gratefully acknowledged.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Rodrı´guez-Iturbe B, Pons H, Herrera-Acosta J, Johnson RJ. The role of immunocompetent cells in non-immune renal diseases. Kidney Int. 2001;59:1626–1640.
- Miller III ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46.
- Reed LJ. A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes. J Biol Chem. 2001;276:38329–38336.
- Tastekin N, Aydogdu N, Dokmeci D, . Protective effects of L-carnitine and alpha-lipoic acid in rats with adjuvant arthritis. Pharmacol Res. 2007;56:303–310.
- Bilska A, Wodek L. Lipoic acid – the drug of the future? Pharmacol Rep. 2005;57:570–577.
- Özler M, Ersöz N, Özerhan İH, Topal T, Öter Ş, Korkmaz A. The effect of alpha-lipoic acid in the prevention of peritoneal adhesions. Turk J Gastroenterol. 2011;22:190–194.
- Tunc T, Oter S, Güven A, . Protective effect of sulfhydryl-containing antioxidants against ischemia/reperfusion injury of prepubertal rat intestine. J Gastroenterol Hepatol. 2009;24: 681–687.
- Guven A, Tunc T, Topal T, . Alpha-lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine. Surg Today. 2008;38:1029–1035.
- Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic Acid. Front Pharmacol. 2011;2:69.
- Melhem MF, Craven PA, Liachenko J, DeRubertis FR. Alphalipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J Am Soc Nephrol. 2002;13:108–116.
- Bhatti F, Mankhey RW, Asico L, Quinn MT, Welch WJ, Maric C. Mechanisms of antioxidant and pro-oxidant effects of alpha-lipoic acid in the diabetic and nondiabetic kidney. Kidney Int. 2005;67:1371–1380.
- Tain YL, Freshour G, Dikalova A, Griendling K, Baylis C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the 5/6 nephrectomized rat. Am J Physiol Renal Physiol. 2007;292:F1404–F1410.
- Lateef H, Aslam MN, Stevens MJ, Varani J. Pretreatment of diabetic rats with lipoic acid improves healing of subsequently-induced abrasion wounds. Arch Dermatol Res. 2005;297:75–83.
- Tanaka T, Kojima I, Ohse T, . Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest. 2005;85:1292–1307.
- Jiang SH, Liu CF, Zhang XL, . Renal protection by delayed ischemic preconditioning is associated with inhibition of the inflammatory response and NF-kappaB activation. Cell Biochem Funct. 2007;25:335–343.
- Budisavljevic MN, Hodge L, Barber K, Fulmer JR, Durazo-Arvizu RA, Self SE. Oxidative stress in the pathogenesis of experimental mesangial proliferative glomerulonephritis. Am J Physiol Renal Physiol. 2003;285:F1138–F1148.
- Quiroz Y, Ferrebuz A, Romero F, Vaziri ND, Rodriguez-Iturbe B. Melatonin ameliorates oxidative stress, inflammation, proteinuria, and progression of renal damage in rats with renal mass reduction. Am J Physiol Renal Physiol. 2008;294:F336–F344.
- Podjarny E, Hasdan G, Bernheim J, . Effect of chronic tetrahydrobiopterin supplementation on blood pressure and proteinuria in 5/6 nephrectomized rats. Nephrol Dial Transplant. 2004;19:2223–2227.
- Anjaneyulu M, Chopra K. Effect of irbesartan on the antioxidant defence system and nitric oxide release in diabetic rat kidney. Am J Nephrol. 2004;24:488–496.
- Alican I, Kaçmaz A, Sakarcan A, Sener G, Paskaloğlu K, Satiroglu H. L-carnitine ameliorates oxidative damage due to chronic renal failure in rats. J Cardiovasc Pharmacol. 2004;43:698–705.
- Sehirli O, Sener E, Cetinel S, Yuksel M, Gedik N, Sener G. Alpha-lipoic acid protects against renal ischemia-reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2008;35:249–255.
- Celik I, Cihangiroglu M, Ilhan N, Akpolat N, Akbulut HH. Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic Clin Pharmacol Toxicol. 2005;97:325–332.
- El-Beshbishy HA, Bahashwan SA, Aly HA, Fakher HA. Abrogation of cisplatin-induced nephrotoxicity in mice by alpha lipoic acid through ameliorating oxidative stress and enhancing gene expression of antioxidant enzymes. Eur J Pharmacol. 2011;668:278–284.
- Lee YM, Bae SY, Won NH, Pyo HJ, Kwon YJ. Alpha-lipoic acid attenuates cisplatin-induced tubulointerstitial injuries through inhibition of mitochondrial bax translocation in rats. Nephron Exp Nephrol. 2009;113:e104–e112.
- Kang KP, Kim DH, Jung YJ, . Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol Dial Transplant. 2009;24: 3012–3020.
- Bae EH, Lee KS, Lee J, . Effects of alpha-lipoic acid on ischemia-reperfusion-induced renal dysfunction in rats. Am J Physiol Renal Physiol. 2008;294:F272–F280.
- Amudha G, Josephine A, Sudhahar V, Varalakshmi P. Protective effect of lipoic acid on oxidative and peroxidative damage in cyclosporine A-induced renal toxicity. Int Immunopharmacol. 2007;7:1442–1449.
- Vaziri ND, Dicus M, Ho N, Sindhu R. Oxidative stress and dysregulation of superoxide dismutase and NAD(P)H oxidase in renal insufficiency. Kidney Int. 2003;63:179–185.
- Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Effect of chronic antioxidant therapy with superoxide dismutase-mimetic drug, tempol, on progression of renal disease in rats with renal mass reduction. Nephron Exp Nephrol. 2009;112:e31–e42.
- Van den Branden C, Verelst R, Vamecq J, Vanden Houte K, Verbeelen D. Effect of vitamin E on antioxidant enzymes, lipid peroxidation products and glomerulosclerosis in the rat remnant kidney. Nephron. 1997;76:77–81.
- Hahn S, Krieg Jr RJ, Hisano S, . Vitamin E suppresses oxidative stress and glomerulosclerosis in rat remnant kidney. Pediatr Nephrol. 1999;13:195–198.
- Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004;11:1135–1146.
- Changchun C, Xiaoqiang D, Zhouluo O, Peng L, Jianzhou Z. In vivo transfection of NF-kappa B decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney Int. 2004;65:834–845.
- Tylicki L, Rutkowski B, Hörl WH. Antioxidants: A possible role in kidney protection. Kidney Blood Press Res. 2003;26:303–314.
- Vaziri ND, Oveisi F, Ding Y. Role of increased oxygen free radical activity in the pathogenesis of uremic hypertension. Kidney Int. 1998;53:1748–1754.
- Vasdev S, Gill VD, Parai S, Gadag V. Effect of moderately high dietary salt and lipoic acid on blood pressure in Wistar-Kyoto rats. Exp Clin Cardiol. 2007;12:77–81.
- Louhelainen M, Merasto S, Finckenberg P, Lapatto R, Cheng ZJ, Mervaala EM. Lipoic acid supplementation prevents cyclosporine-induced hypertension and nephrotoxicity in spontaneously hypertensive rats. J Hypertens. 2006;24:947–956.