Abstract
Aims: Intercellular adhesion molecule-1 (ICAM-1) plays an important role in the inflammatory process and immune response. The aim of the study was to investigate ICAM-1 expression in the kidneys of spontaneously hypertensive rats (SHRs) and the relationship between the level of ICAM-1 and renal damage. Methods: Male Wistar–Kyoto (WKY) rat and SHR models were employed. Blood pressure (BP) was recorded using tail cuff method. Twenty-four hour proteinuria and β2-microglobulin (β2-MG) were measured using biuret method and radioimmunity kits, respectively. Biochemical parameters were measured after the animals were killed. ICAM-1 expression in renal tissue was assessed using western blot and real-time polymerase chain reaction (PCR). Results: It was observed that BP, proteinuria, and urine β2-MG were more increased in SHR groups than that in the same age WKY groups. In SHR groups, BP, proteinuria, and urine β2-MG at the 56th week were significantly higher than that at the 28th week. ICAM-1 protein and mRNA expression in SHR renal tissues was significantly increased in SHR groups compared with the same age in WKY rats. ICAM-l expression was positively correlated with proteinuria and urine β2-MG. Conclusion: This study demonstrated that the renal expression of ICAM-1 was increased in SHR with renal damage, and inflammation may be involved in the hypertensive renal damage.
INTRODUCTION
A large body of research over the past decade revealed that kidney is one of the major target organs in essential hypertension.Citation1 The pathogenic mechanism of hypertensive nephropathy is complicated and inflammation may be involved in the development and progression of hypertensive renal damage.Citation2–4 Intercellular adhesion molecule-1 (ICAM-1), a member of the immunoglobulin superfamily expressed on the cell surface of a wide variety of cell types, is one of the key molecules to mediate the inflammatory process and immune response.Citation5,6 Therefore, we investigated the expression of ICAM-1 in the kidneys of spontaneously hypertensive rats (SHR) and the relationship between the level of ICAM-1 and renal damage.
MATERIALS AND METHODS
Male rats were maintained in the animal facility of Nantong University Medical Center, where they were housed in a constant-temperature room with a 12-h dark/12-h light circle and allowed free access to standard rodent chow and water. Male Wistar–Kyoto (WKY) rats aged 8 weeks (weight 180–200 g) were obtained from Beijing Hua Tong Animal Research Center. Male SHR aged 8 weeks (weight 180–200 g) were obtained from Shanhai SLAC Laboratory Animal Breeding Center. Four groups of rats were studied: 28w-WKY group (n = 9); 56w-WKY group (n = 9); 28w-SHR group (n = 9); and 56w-SHR group (n = 9). All animal studies were approved by the Institutional Animal Care and Use Committees of Nantong University. Rats were killed at the end of week 28 or 56. Kidney specimens were harvested for histopathology, immunohistochemistry, western blot, and real-time polymerase chain reaction (PCR), and serum samples were collected for biochemical tests.
URINE AND SERUM BIOCHEMICAL TESTS
The 24-h urine samples were collected in metabolism cages and 24-h urine protein excretion and β2-microglobulin (β2-MG) were determined using biuret method and radioimmunity kits, respectively. Arteria caudalis pressure was recorded using tail-cuff method (RBP-1, Beijing, China). Immediately before euthanasia, blood samples were drawn from the aorta. Blood urea nitrogen (BUN) and serum creatinine (Scr) were determined in all blood samples using standard diagnostic kits.
LIGHT MICROSCOPY
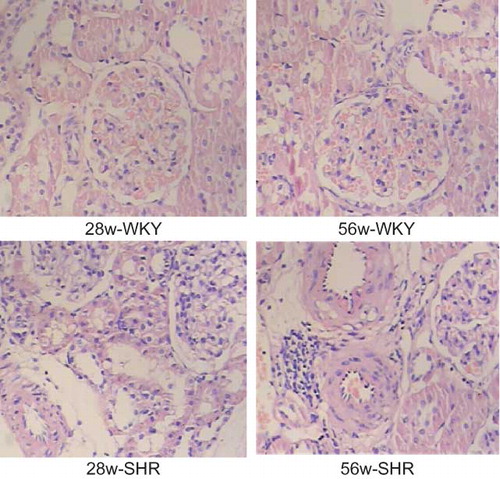
The kidney fragments were fixed with 4% paraformaldehyde/phosphate-buffered saline (PBS) solution dehydrated and embedded in paraffin. Sections (2 μm thick) were stained with hematoxylin–eosin (HE). A light microscope (Olympus, Tokyo, Japan) was utilized to evaluate the morphological changes.
IMMUNOHISTOCHEMISTRY ANALYSIS FOR ICAM-1
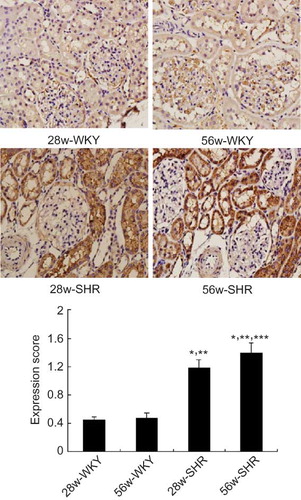
For immunohistochemistry examination, kidney sections were immunostained using immunoperoxidase technique with Vector ABC kit (Vector Laboratories, Burlingame, CA, USA). Briefly, sections (3 μm thick) were blocked with 3% bovine serum albumin for 30 min at room temperature and incubated overnight at 4°C with the rabbit anti-ICAM antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Sections were then washed and incubated with biotinylated secondary antibodies for 60 min at room temperature. Biotin was identified and visualized with 3,3-diaminobenzidine solution (DAB). Counterstaining was then performed before examination under a light microscope. Random 100 glomeruli from each renal specimen were observed, and images were then analyzed with Image Pro Plus 6.0 edition (Media Cybernetics, Bethesda, MD, USA) for the determination of immunostained area. The percentage of the stained area was calculated as the ratio of suitable binary threshold image and the total field area.
RNA EXTRACTION AND QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION
Total RNA was extracted from renal cortex tissue using TRIzol reagent (Invitrogen, San Diego, CA, USA) according to the manufacturer’s instructions. RNA concentration and quality were assessed using spectrophotometry at wavelengths of 260 and 280 nm and using agarose gel electrophoresis. Reverse transcription was performed using a first-strand cDNA synthesis kit (Fermentas Life Sciences St. Leon-Rot, Germany). Primers for β-actin and ICAM-1 were designed and synthesized by Shanghai Sangong Gene Corporation. Real-time PCR was performed using an SYBR Green/ROX qPCR Master Mix kit (Fermentas Life Sciences) and the Rotor-Gene-3000A Realtime PCR System (Corbett, Sydney, Australia), according to the manufacturer’s protocols. The primer sequences used were as follows: ICAM-1 (forward, 5′-CCTGGGTCATAATTGTTGGTG-3′; reverse, 5′-AGGAAGTCAGCCTTTCTTGG-3′) and β-actin (forward, 5′-CCCATACCCACCATCACACC-3′; reverse, 5′-GAGAGGGAAATCGTGCGTGAC-3′). All samples for each gene were run in duplicate. Gene expression values were calculated on the basis of the comparative threshold cycle (Ct) method, normalized to the expression values of β-actin, and displayed as fold induction relative to control.
WESTERN BLOT ANALYSIS
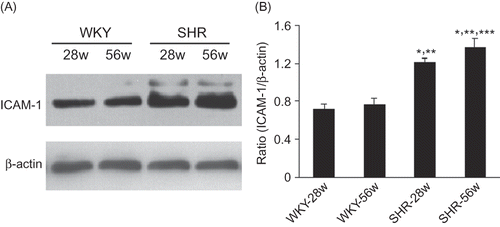
Protein concentration was determined using the bicinchoninic acid protein assay (Sigma-Aldrich, St. Louis, MO, USA). Fifty micrograms of proteins was loaded in each lane of a 10% SDS-PAGE gel and run at 100V. Protein was transferred to nitrocellulose membranes, which were then washed and incubated with blocking buffer (5% non-fat milk in PBS containing 0.1% Tween 20) for 1 h at room temperature. The membranes were then incubated overnight at 4°C with the primary antibodies: rabbit anti-ICAM-1 antibody (Santa Cruz Biotechnology) and mouse anti-β-actin antibody (Sigma). After three washes, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature followed by further washes. Antibody labeling was visualized via ECL (Amersham Biosciences, Little Chalfont, UK).
STATISTICAL ANALYSIS
Statistical software SPSS ver. 15.0 (SPSS, Chicago, IL, USA) was used to perform data statistical analysis. Data were shown as mean ± standard deviation. Statistical significance was determined using one-way ANOVA. Differences with p < 0.05 were considered statistically significant.
RESULTS
Arteria Caudalis Pressure, Renal Function, 24-h Proteinuria, and 2-MG
As shown in , there was no significant difference in arteria caudalis pressure, 24-h proteinuria and β2-MG between 28w- and 56w-WKY groups. However, the measurements were higher in the 28w-SHR group than that in the 28w-WKY group. Compared with the 28w-SHR group, 56w-SHR group induced a significant increase in blood pressure (BP), 24-h proteinuria, and β2-MG. There was no significant difference in BUN and Scr either within or between the four groups.
Table 1. Blood pressure and biochemical data from SHR and WKY rats.
Renal Tissue Damage
As shown in , no histologic abnormalities of the kidneys were observed at the light microscopic level in the WKY group. Compared with the WKY group, 28w-SHR group showed hypertrophy and hyperplasia of medial smooth muscle cells, vessel wall thickening, intimal hyperplasia, vessel lumen stenosis, mild inflammatory cell infiltration, mesangial cell proliferation, and tubular cloudy swelling. The 56w-SHR group showed a progressive increase in pathologic lesions, including a moderate inflammatory cell infiltration, segmental glomerular sclerosis, and tubular atrophy.
ICAM-1 Expression in Immunohistochemistry
As shown in , ICAM-1 was slightly expressed in glomerular mesangial cells, epithelial cells, and vascular endothelial cells in WKY groups. In the 28w-SHR group, a marked increased expression of ICAM-1 was observed in the mesangial cells, renal capsule epithelial cells, tubular epithelial cells and renal artery endothelium, and adventitia. The increase in renal tubular cells was also more prominent in the 56w-SHR group than that in the 28w-SHR group.
Figure 2. Immunohistochemical micrographs of ICAM-I staining in the kidneys of 28w-WKY, 56w-WKY, 28w-SHR, and 56w-SHR (original magnification ×400). Renal tissue expression of ICAM-1 was scored using four levels, and average value was obtained from analyses of 100 glomeruli in each group. Data are shown as means ± SD.
Notes: *p < 0.05 versus 28w-WKY rats; **p < 0.05 versus 56w-WKY rats; ***p < 0.05 versus 28w-SHR rats.
The Relationship between 24-h Proteinuria, β2-MG, and ICAM-1 Expression in the Kidney
There was no significantly difference between 28w-WKY and 56w-WKY groups in proteinuria, β2-MG, and ICAM-1 expression. With the extension of the duration of hypertension, 24-h proteinuria and β2-MG were significantly increased and ICAM-1 expression also increased in kidney. The expression of ICAM-1 was positively correlated with 24-h proteinuria, β2-MG, and BP in 28w-SHR group (r24hUpro = 0.829, p < 0.05; rβ2-MG = 0.614, p < 0.05; rBP = 0.946, p < 0.05). Moreover, ICAM-1 expression was positively correlated with 24-h proteinuria and β2-MG in 56w-SHR group (r24hUpro = 0.913, p < 0.05; rβ2-MG = 0.735, p < 0.05), but no relationship with BP.
ICAM-1 mRNA Expression
Because we found differences in the renal tissue damage in different groups, we further studied the expression of ICAM-1 mRNA genes by real-time PCR. Weak expression was detected in the 28w-WKY and 56w-WKY groups with no significant difference. The gene expression of ICAM-1 was significantly increased in the kidneys of the 28w-SHR group, especially in 56w-SHR group ().
Figure 3. Renal mRNA expression of ICAM-1 in four groups. The relative expression ratio of ICAM-1 mRNA in relation to β-actin mRNA was determined. Data are shown as means ± SD.
Notes: *p < 0.05 versus 28w-WKY rats; **p < 0.05 versus 56w-WKY rats; ***p < 0.05 versus 28w-SHR rats.
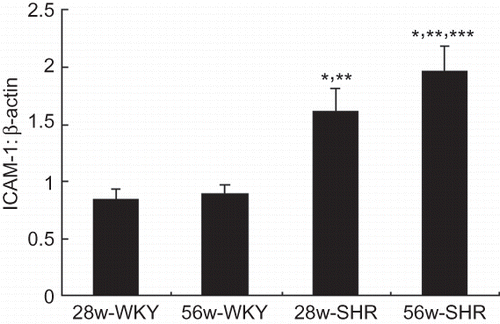
Figure 4. Western blot analysis shows that SHR promotes ICAM-1 expression. (A) Kidney lysates of 28w and 56w kidneys from WKY and SHR rats were examined using western blotting for ICAM-1 and reprobed for β-Actin as a loading control. (B) Semi-quantitative analysis of ICAM-1. Data are shown as means ± SD.
Notes: *p < 0.05 versus 28w-WKY rats; **p < 0.05 versus 56w-WKY rats; ***p < 0.05 versus 28w-SHR rats.
ICAM-1 Protein Expression
As shown in , western blot analysis revealed that compared with the WKY groups, ICAM-1 were significantly upregulated in SHR groups. Compared with 28w-SHR group, the expression of ICAM-1 was significantly increased in the kidneys of 56w-SHR group.
DISCUSSION
It is generally accepted that the spontaneously hypertensive rat represents an analogue of human essential hypertension.Citation7,8 Long-term high BP can induce secondary hypertensive renal damage, whose pathological features are similar to human hypertensive renal damage.Citation9,10 Hypertension could increase glomerular hyperfiltration and damage the filtration membrane barrier, which can increase urinary albumin excretion and even massive proteinuria.Citation11–13 Therefore, the early diagnosis of hypertensive renal damage was mainly based on elevated proteinuria that reflects the change of hemodynamics and the damage of vascular endothelial function.Citation14–16 Hypertension also could lead to renal tubular reabsorption and decomposition dysfunction and increase urinary β2-MG excretion, which is a sensitive marker reflecting the proximal renal tubular dysfunction.Citation17 This study demonstrated that tail artery pressure, proteinuria, and urinary β2-MG were significantly increased in the 28w-SHR group compared with that in the WKY groups, especially in the 56w-SHR group. Renal pathology showed the characteristic changes of hypertensive renal damage. Immunohistochemistry, real-time PCR, and western blot showed only slightly ICAM-1 expression in WKY kidney tissue. However, with the increase in proteinuria and β2-MG in SHR group, the significantly elevated ICAM-1 expression in kidney tissue, especially in renal tubular epithelial cells, suggested that ICAM-1 participated in the process of renal damage. Hypertension led to kidney injury which started the process of inflammation. It is suggested that inflammation participated in the occurrence of hypertensive renal damage.
ICAM-1 belongs to a member of cell adhesion molecules in the immunoglobulin superfamily, which is expressed on the cell surface of a wide variety of cell types. The level of ICAM-1 expression is partly dependent on the availability of specific receptor-mediated signal transduction pathways and their nuclear transcription factor targets on the ICAM-1 promotor.Citation18 Through binding its receptor, it could mediate monocytes, lymphocytes, and leukocytes adhering to endothelial cells, then leukocyte exudation and tissue infiltration.Citation19 Therefore, ICAM-1 is a key molecule to mediate the inflammatory process and the immune response.Citation20–22 Hypertension, an initial factor in vascular endothelial cell injury, can activate the renin–angiotensin system. Angiotensin II can activate the transcription factor NF-κB, which increases the expression of ICAM-1 through the cascade.Citation23–25 Moreover, C-reactive protein and oxygen-free radicals, which play important roles in the pathology of hypertension, are able to induce ICAM-1 expression through NF-κB-dependent pathways.Citation26 Increased ICAM-1 expression can mediate a large number of leukocyte adhesions to endothelium, which can release more inflammatory mediators and damage endothelial cells. Endothelial dysfunction including vasoconstriction, stenosis, and vascular remodeling could affect microcirculation, aggravate hypertensive condition, and cause target organ damage.Citation27–29 In normal kidney, ICAM-1 is expressed in low levels by endothelial cells of large vessels, peritubular capillaries, mesangial cells, brush border of proximal tubules, epithelial cells of Bowman’s capsule, and some interstitial cells, which is similar to our study.Citation30,31 This study demonstrated that ICAM-1 was abundantly expressed in glomeruli, tubules, and vascular endothelial in SHR with renal damage, which was related to the infiltration multinucleated cells in the same area. ICAM-1 involved in the development and progression of renal fibrosis. Through binding to its receptor, ICAM-1 can mediate monocytes, lymphocytes, and neutrophils to adhesion and aggregation in tubular and interstitial cells. Inflammatory leukocytes could secrete a variety of profibrogenic factors, activate fibroblasts, produce extracellular matrix, and slow down the degradation of the extracellular matrix. All of these can increase collagen synthesis, extracellular matrix accumulation, cell proliferation, and renal fibrosis. The accumulation of extracellular matrix also can further induce these cells to produce inflammatory cytokines such as ICAM-1 to promote the development of kidney disease.Citation32–35
In this study, we also found that renal ICAM-1 expression was positively correlated to 24-h proteinuria and β2-MG in 28w- and 56w-SHR groups. However, due to the BP in SHR after 20 weeks was almost close to the peak, there was no correlation between renal ICAM-1 expression and BP in 56w-SHR group. With the persistence of high BP and renal damage, proteinuria and urinary β2-MG were aggravated. Therefore, it further illustrated that the expression of ICAM-1 was closely related with kidney damage. Tesar and Abd-Elkareem also demonstrated that lupus nephritis patients with advanced renal damage have high urinary levels of ICAM-1 when compared with SLE without nephritis.Citation36,37
Several studies in recent years partially confirmed that inflammation is closely correlated to hypertension.Citation38–40 However, there were few studies about inflammation effect on hypertensive renal damage. This study demonstrated that the renal expression of ICAM-1 was increased in SHR with renal damage, and inflammation may play an important role in the development of hypertensive renal damage.
ACKNOWLEDGMENTS
The authors thank Dr. Zhengping Pan for his technical assistance and his skilful assistance in handling experimental animals. This work was supported by grants from Nantong Natural Science Foundation (no. S2009062).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Ono H, Ono Y. Nephrosclerosis and hypertension. Med Clin North Am. 1997;81:1273–1288.
- Feld LG, Van Liew JB, Galaske RG, Boylan JW. Selectivity of renal injury and proteinuria in the spontaneously hypertensive rat. Kidney Int. 1977;12:332–343.
- Hilgers KF, Klanke B, Cordasic N, Hartner A, Schmieder RE, Veelken R. Blood pressure versus direct mineralocorticoid effects on kidney inflammation and fibrosis in DOCA-salt hypertension. Nephrol Dial Transplant. 2008;23:3456–3463.
- Wang PX, Sanders PW. Mechanism of hypertensive nephropathy in the Dahl/Rapp rat: A primary disorder of vascular smooth muscle. Am J Physiol Renal Physiol. 2005;288:F236–F42.
- Paletas K, Boura P, Magoula I, Alexiadis S, Tsapas A. Highly increased ICAM-1 levels in essential hypertension: Correlation to proinflammatory cytokines levels. Am J Hypertens. 1999;12:66a–66a.
- Buemi M, Marino D, Floccari F, . Effect of interleukin 8 and ICAM-1 on calcium-dependent outflow of K(+) in erythrocytes from subjects with essential hypertension. Curr Med Res Opin. 2004;20:19–24.
- Zhou XJ, Vaziri ND, Zhang J, Wang HW, Wang XQ. Association of renal injury with nitric oxide deficiency in aged SHR: Prevention by hypertension control with AT1 blockade. Kidney Int. 2002;62:914–921.
- Chung S, Park CW, Shin SJ, . Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol Dial Transplant. 2010;25:389–399.
- Pravenec M, Kajiya T, Zidek V, . Effects of human C-reactive protein on pathogenesis of features of the metabolic syndrome. Hypertension. 2011;57:731–737.
- Ito Y, Aten J, Nguyen TQ, . Involvement of connective tissue growth factor in human and experimental hypertensive nephrosclerosis. Nephron Exp Nephrol. 2011;117:e9–e20.
- Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: A viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res. 2009;32:115–121.
- Palatini P, Mormino P, Dorigatti F, . Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: The HARVEST. Kidney Int. 2006;70:578–584.
- Schillaci G, Pirro M, Pucci G, . Is glomerular hyperfiltration a correlate of the metabolic syndrome in never-treated essential hypertension? J Hypertens. 2009;27:S401–S401.
- Garg JP, Bakris GL. Microalbuminuria: Marker of vascular dysfunction, risk factor for cardiovascular disease. Vasc Med. 2002;7:35–43.
- van Dokkum RPE, Ochodnicky P, Henning RH, Buikema HJ, de Zeeuw D, Provoost AP. Renal vascular dysfunction precedes the development of renal damage in the hypertensive Fawn-Hooded rat. Am J Physiol Renal Physiol. 2010;298:F625–F633.
- Imig JD, Knight SF, Yuan JH, Roy S. Simvastatin and tempol protect against endothelial dysfunction and renal injury in a model of obesity and hypertension. Am J Physiol Renal Physiol. 2010;298:F86–F94.
- Zhong J, Guo D, Chen CB, . Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57:314–322.
- Niessen HM, Krijnen PA, Visser CA, Meijer CJ, Hack CE. Intercellular adhesion molecule-1 in the heart. Ann N Y Acad Sci. 2002;973:573–585.
- Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32.
- Pickering TG. Stress, inflammation, and hypertension. J Clin Hypertens. 2007;9:567–571.
- Frank PG, Lisanti MP. ICAM-1: Role in inflammation and in the regulation of vascular permeability. Am J Physiol Heart Circ Physiol. 2008;295:H926–H927.
- Witkowska AM. Soluble ICAM-1: A marker of vascular inflammation and lifestyle. Cytokine. 2005;31:127–134.
- Spencer CG, Martin SC, Felmeden DC, Blann AD, Beevers GD, Lip GY. Relationship of homocysteine to markers of platelet and endothelial activation in “high risk” hypertensives: A substudy of the Anglo-Scandinavian Cardiac Outcomes Trial. Int J Cardiol. 2004;94:293–300.
- Kashyap MK, Yadav V, Sherawat BS, . Different antioxidants status, total antioxidant power and free radicals in essential hypertension. Mol Cell Biochem. 2005;277:89–99.
- Polivoda SN, Cherepok AA. Changes in activity of transcription factors in the cells of vascular endothelium as a pathological mechanism of its dysfunction in essential hypertension. Ter Arkh. 2005;77:59–62.
- Tong S, Neboori HJ, Tran ED, Schmid-Schönbein GW. Constitutive expression and enzymatic cleavage of ICAM-1 in the spontaneously hypertensive rat. J Vasc Res. 2011;48:386–396.
- Mills PJ, von Kanel R, Hong S, Pung MA. Association of blood pressure and fitness with levels of atherosclerotic risk markers pre-exercise and post-exercise. Am J Hypertens. 2007;20:670–675.
- Madej A, Okopien B, Kowalski J, Haberka M, Herman ZS. Plasma concentrations of adhesion molecules and chemokines in patients with essential hypertension. Pharmacol Rep. 2005;57:878–881.
- Glowinska B, Urban M, Peczynska J, Florys B. Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension, and diabetes. Metabolism. 2005;54: 1020–1026.
- Lhotta K, Neumayer HP, Joannidis M, Geissler D, Konig P. Renal expression of intercellular-adhesion molecule-1 in different forms of glomerulonephritis. Clin Sci. 1991;81:477–481.
- Hussein MRA, Abd-Elkareem MI, Al Tamimy HM, Khamis OA, Abdellatif SS. Increased urinary levels of the leukocyte adhesion molecules ICAM-1 and VCAM-1 in human lupus nephritis with advanced renal histological changes: Preliminary findings. Clin Exp Nephrol. 2010;14:548–557.
- Chen XM, Cai GY, Zhang XG, . Tissue inhibitor of metalloproteinase-1 exacerbated renal interstitial fibrosis through enhancing inflammation. Nephrol Dial Transplant. 2008;23:1861–1875.
- Chen XM, Zhang XG, Hong Q, . TIMP-1 promotes age-related renal fibrosis through upregulating ICAM-1 in human TIMP-1 transgenic mice. J Gerontol A Biol Sci Med Sci. 2006;61:1130–1143.
- Steadman R, Clayton A. ICAM-1 interactions in the renal interstitium: A novel activator of fibroblasts during nephritis. Histol Histopathol. 1999;14:861–870.
- Lan HY, Chen HY, Huang XR, . The protective role of Smad7 in diabetic kidney disease: Mechanism and therapeutic potential. Diabetes. 2011;60:590–601.
- Tesar V, Masek Z, Rychlík I, . Cytokines and adhesion molecules in renal vasculitis and lupus nephritis. Nephrol Dial Transplant. 1998;13:1662–1667.
- Abd-Elkareem MI, Al Tamimy HM, Khamis OA, Abdellatif SS, Hussein MR. Increased urinary levels of the leukocyte adhesion molecules ICAM-1 and VCAM-1 in human lupus nephritis with advanced renal histological changes: Preliminary findings. Clin Exp Nephrol. 2010;14:548–557.
- Montecucco F, Pende A, Quercioli A, Mach F. Inflammation in the pathophysiology of essential hypertension. J Nephrol. 2011;24:23–34.
- Katsi V, Tsartsalis D, Kontoangelos K, . Inflammation and essential hypertension: A diptych which affects quality of life. J Hypertens. 2010;28:E267–E267.
- Bilovol O. Markers of systemic inflammation in essential hypertension. J Hypertens. 2009;27:S370–S371.