Abstract
Introduction: This study was designed to investigate the possible beneficial effects of medical ozone therapy (OT), known as an immunomodulator and antioxidant, on the renal function, morphology, and biochemical parameters of oxidative stress in kidneys subjected to ischemia/reperfusion injury (IRI). Materials and methods: Thirty male Sprague–Dawley rats were classified into three groups: control, renal IRI, and renal IRI + OT. The IRI group was induced by bilateral renal ischemia for 60 min, followed by reperfusion for 6 h. After reperfusion, the kidneys and blood of rats were obtained for histopathologic and biochemical evaluation. Results: Renal IRI increased the tissue oxidative stress parameters (lipid peroxidation, protein oxidation, and nitrite plus nitrate) and decreased the antioxidant enzyme activities (superoxide dismutase and glutathione peroxidase). The serum neopterin levels showed correlation with oxidative stress parameters. All these parameters were brought to control values in the treatment group. Histopathologically, the kidney injury in the treatment group was significantly lesser than in the renal IRI group. Conclusions: Our results clearly showed that OT has beneficial effect to protect kidney against IRI. The serum neopterin levels might be used as a marker to detect the degree of renal IRI.
INTRODUCTION
Ischemia (cessation of blood flow), followed by reperfusion (reestablishment of blood flow), causes morphological and functional damages, which increases during the reperfusion phase. Reperfusion of kidney increases the effects of early ischemic injury by release of oxygen-derived reactive oxygen species (ROS), such as superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (•OH), or nitrogen-derived reactive nitrogen species (RNS), such as nitric oxide (NO) and peroxynitrite (ONOO−).Citation1–3 The consequences of oxidative and nitrosative stress are multiple and include lipid peroxidation, resulting in the destruction of membrane lipids and oxidative DNA damage, collectively leading to the loss of cell viability, either by necrotic or apoptotic pathways.Citation1–3
The intracellular and molecular mechanisms involved in the development of renal ischemia/reperfusion injury (IRI) regarding ROS/RNS are complex and not yet fully understood, but have been extensively discussed and reviewed. Several investigations have demonstrated the beneficial effects of pharmacological interventions, which prevent ROS generation, inhibit the enzymes responsible for ROS generation, and in administration of antioxidant enzymes, which degrade ROS and ROS scavenging molecules.Citation4–8
Neopterin is synthesized from guanosine triphosphate in macrophages and monocytes.Citation9 Urine and serum neopterin levels reflect the activation of the macrophages/monocytes.Citation10 Neopterin is one of the useful tools to assess the cell-mediated immune response and has been accepted as strong indicator for the clinical severity of some diseases.Citation9,11–13
A gas mixture comprising ozone/oxygen used in medicine is known as medical ozone therapy (OT). Ozone/oxygen mixture exhibits various effects on the immune system, such as in the modulation of phagocytic activity of peritoneal and alveolar macrophages.Citation14–16 Clinical studies have so far shown that OT appears useful in diseases, including peritonitis, infected wounds, and advanced ischemic diseases.Citation16,17 It was also demonstrated that ozone increases antioxidant enzyme activities such as glutathione peroxidase (GSH-Px), SOD, and catalase (CAT), preparing the host to face physiopathological conditions mediated by ROS/RNS.Citation15,18 Therefore, a study has been designed to evaluate the possible beneficial effects of medical OT on kidney damage induced by renal IRI.
MATERIALS AND METHODS
Animals and Surgery Procedure
The project was approved by the local Experimental Ethics Committee and the National Institute of Health’s Guide for the Care and Use of Laboratory Animals was followed. Before experiment, animals were fed standard rat chow and water ad libitum and housed in cages with controlled temperature and 12-h light/dark cycle for at least 1 week.
Thirty male Sprague–Dawley rats, weighing 200–250 g and aged 2–2.5 months each, were randomly divided into three groups: control, renal IRI, and renal IRI + OT. Following a 12-h fasting period, the animals were anesthetized with an intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg). A midline incision was made, the renal pedicle was observed, and the arteries were bilaterally occluded using an atraumatic microvascular clamp (Bulldog Artery Clamp; Harvard Apparatus, Holliston, MA, USA) for 60 min. After 60 min of renal ischemia, clamps were removed and the kidneys were inspected for restoration of blood flow and the abdomen was closed. Sham-operated animals underwent the same surgical procedure without clamp application. Following 6 h of reperfusion period, animals were killed by cervical dislocation. At the time of death, blood was collected by heart puncture for measurement of biochemical analysis. Both kidneys were harvested for histopathological evaluation and biochemical examination.
The rats in the renal IRI + OT group were administrated ozone/oxygen mixture at a single dose of 0.7 μg/kg via intraperitoneal route after closing the abdominal wall.
Ozone (O3) was generated by the ozone generator (OZONOSAN Photonik 1014, Hansler GmbH, Nordring 8, Iffezheim, Germany), allowing control of the gas flow rate and ozone concentration in real time by a built-in UV spectrometer. The ozone flow rate was kept constant at 3 L/min, representing a concentration of 60 mg/mL and gas mixture of 97% O2 + 3% O3. Tygon polymer tubes and single-use silicon-treated polypropylene syringes (ozone resistant) were used throughout the reaction to ensure containment of O3 and consistency of concentrations.
Biochemical Analysis
Serum samples were used for the measurement of blood urea nitrogen (BUN) and serum creatinine (SCr) levels, which were used as indicators of impaired glomerular function, and aspartate aminotransferase (AST), which was used as an indicator of renal IRI.Citation19 BUN, SCr, and AST were determined with an Olympus AU 2700 autoanalyzer (Olympus, Hamburg, Germany) using original kits.
The frozen tissues were homogenized in phosphate buffer (pH 7.4) by means of a homogenizator (Heidolph Diax 900; Heidolph Elektro GmbH, Kelheim, Germany) in an ice cube. Homogenates were centrifuged at 14,000 rpm (7530× g) in 4°C for 10 min. First of all, the protein content of tissue homogenates was measured using the method of Lowry et al. with bovine serum albumin as the standard. The efficacy of treatment was assessed by tissue levels of malondialdehyde (MDA) using the method of Ohkawa et al., superoxide dismutase (SOD) using the method of Sun et al., and glutathione peroxidase (GPx) using the method of Paglia and Valentine. Protein carbonyl content (PCC) using the method of Levine et al. as has already been described in our previous works.Citation4,5,8,20,21
Samples were analyzed for their nitrate plus nitrite (NOx) concentrations, end products of nitric oxide degradation, by using ion chromatography (Dionex ICS – 1000, Sunnyvale, CA, USA). The supernatant of the tissues was filtered using 22-nm injector filters (Sartorius Minisart, Goettingen, Germany). Selective anion columns and accessories were used (AS-9 HC, AG-9 HC, Sunnyvale, CA, USA). Standardized ion mixtures at different concentrations were used for measuring the nitrate and nitrite quantities of samples.
Serum neopterin levels were measured using a high-pressure liquid chromatography (HPLC) system (Agilent Technologies Inc., 1200 Series System, Santa Clara, CA, USA), with a fluorescence detector as described previouslyCitation22,23 and presented as nmol/L.
Histopathologic Evaluation
Both kidneys of each animal were taken for histopathologic evaluation. In all groups, samples of kidney were placed in formalin and processed through to paraffin. They were subsequently sectioned at 5 μm and stained with hematoxylin–eosin (H&E). The sections were scored with a previously described semiquantitative scale designed to evaluate the degree of renal damage (tubular cell necrosis, cytoplasmic vacuole formation, hemorrhage, and tubular dilatation).Citation24 A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes. The scoring system used was 0, absent; 1, present; and 2, marked. The total histopathologic injury score per kidney was calculated by addition of all scores. Blind analysis of the histological samples was performed by two independent experts.
Statistical Analysis
All biochemical data were expressed as mean ± standard error of the mean (SEM). All statistical analyses were carried out using SPSS statistical software (SPSS Inc., for Windows, Version 15.0, Chicago, IL, USA). The differences in measured parameters among the three groups were analyzed by Kruskal–Wallis test. Dual comparisons between groups that present significant values were evaluated with Mann–Whitney U-test. p-Values of <0.05 were accepted as statistically significant.
RESULTS
The values of biochemical evaluation of serum for each group are shown in . There was a significant increase in SCr and BUN levels in the renal IRI group, suggesting a significant degree of glomerular dysfunction. Renal IRI also produced a significant increase in serum AST levels, which is accepted as a marker of renal injury (p < 0.05). Administration of OT decreased the SCr, BUN, and AST levels (p < 0.05).
Table 1. Biochemical evaluation of serum for each group.
The outcome of oxidative stress parameters are shown in . Administration of OT significantly increased SOD and GSH-Px activities (p < 0.05). The MDA levels and PCC content in the renal tissue are shown in . There was a clear increase in the tissue MDA and PCC content and the NOx levels in the renal IRI group, suggesting increased lipid peroxidation, protein oxidation, and peroxynitrite production (p < 0.05). The MDA, PCC content, and NOx levels in the treatment group were significantly lower than those in the renal IRI group (p < 0.05).
Table 2. Biochemical evaluation of kidney for each group.
Table 3. Histopathologic evaluation of kidney sections for each group.
The serum neopterin levels were significantly increased in the renal IRI group when compared with the control group (p < 0.05). On the contrary, neopterin levels decreased in the treatment group (p < 0.05), still they were higher than control values (p < 0.05).
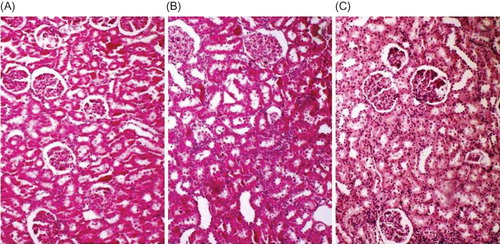
Histopathologic grading of renal injury is displayed as median (min–max) in . The total injury score was significantly increased in the renal IRI group. Contrary to this, total histological injury score was significantly decreased in the treatment group. Representative histological samples from all groups are shown in .
Figure 1. Representative histological photographs of kidney tissues from (A) control, (B) renal IRI, and (C) renal IRI + OT groups. Control group animals show normal histological characteristics of glomeruli and tubules. Rats subjected to renal IRI show marked necrosis with tubular dilation, swelling, luminal congestion, and medullar hemorrhage. Rats subjected to renal IRI injury and treated with OT show moderate kidney damage and moderate dilatation of the tubular structure.
DISCUSSION
Our findings showed that medical OT had an ameliorative effect on both the antioxidant status and the histopathological changes in the kidney subjected to IRI. Our study also demonstrated that renal IRI caused a dramatic increase of kidney neopterin levels.
The highlight of this study is that ozone administration significantly reduced the severity of IRI in rats’ kidneys. After administration, ozone is dissolved in biological fluids such as plasma, lymph, and urine; it immediately reacts with macromolecular glycoproteins comprising the carbohydrates and polypeptide chains, namely proteoglycans and collagen types II and IV. All of these compounds act as electron donors and undergo oxidation, resulting in the formation of H2O2 and lipid oxidation products (LOPs). H2O2, an essential ROS molecule, is able to act as an ozone messenger for eliciting several biological and therapeutic effects.Citation14,15,25,26 In contrast to the conventional idea that H2O2 is harmful, it has been widely accepted that it acts as a regulator of signal transduction and is an important mediator of host defense and immune responses.Citation26,27 Although H2O2 acts immediately and disappears (early and short-acting messenger), LOPs, via the circulation, distribute throughout the tissues and become late and long-lasting messengers.Citation15 This process stimulates the innate immune system and helps the cell to survive when an injury occurs. In addition, it has been demonstrated that ozone supports cellular antioxidant systems, involving glutathione, SOD and catalase, and enzymatic reactions, preparing the host to face physiopathological conditions mediated by ROS/RNS and septic shock.Citation26,28–30 In light of recent pharmacological knowledge, our consideration is that ozone acts as a pro-drug and induces a rearrangement of the biochemical pathways with the activation of a second messenger in a cascade with a multiple system action.Citation31
We also aimed at evaluating the serum neopterin levels of animals. Neopterin is formed during the course of cell-mediated immune response and there is a strong correlation between detection of neopterin and the ability of monocytes/macrophages to scavenge ROS.Citation32 Thus, neopterin determination may be considered as both an indirect marker for the amount of immunologically induced oxidative/nitrosative stress and the effects of therapeutic interventions that are assigned to interfere with the degree of immune activation. According to serum neopterin levels, renal IRI causes immunologically induced oxidative stress and OT is assumed to be effective affecting cell immunity. Contrary to this, serum neopterin level might be used as a renal injury indicator in renal IRI.
There are various studies showing OT is beneficial on different clinical entities such as osteomyelitis, pleural empyema, abscesses with fistulae, infected wounds, advanced ischemic diseases (hind limb ischemia), and heart ischemia.Citation31,33 Recently, we have reported that OT has a preventive effect in the intestine and esophagus by decreasing tissue damage and increasing the antioxidant enzyme activity in experimental model of distal colitis, necrotizing enterocolitis, caustic esophageal burn model, acute necrotizing pancreatitis, and acetaminophen-induced nephrotoxicity.Citation20,34–38
In conclusion, our data suggest that OT might protect kidneys against IRI by modulating a moderate oxidative and nitrosative stress, which, in turn, increases the antioxidant endogenous systems.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
REFERENCES
- Sheridan AM, Bonventre JV. Pathophysiology of ischemic acute renal failure. Contrib Nephrol. 2001;132:7–21.
- Greene EL, Paller MS. Oxygen free radicals in acute renal failure. Miner Electrolyte Metab. 1991;17:124–132.
- Yaman H, Cayci T, Seyrek M, et al. Effects of vitamin A and C and melatonin on 3-nitrotyrosine formation in guinea pig heart under lipopolysaccharide-induced stress. Turk J Med Sci. 2010;40:715–721.
- Ersoz N, Guven A, Cayci T, . Comparison of the efficacy of melatonin and 1400 W on renal ischemia/reperfusion injury: A role for inhibiting iNOS. Ren Fail. 2009;31:704–710.
- Guven A, Uysal B, Akgul O, . Scavenging of peroxynitrite reduces renal ischemia/reperfusion injury. Ren Fail. 2008;30: 747–754.
- Kizilgun M, Poyrazoglu Y, Oztas Y, . Beneficial effects of N-acetylcysteine and ebselen on renal ischemia/reperfusion injury. Ren Fail. 2011;33:512–517.
- Oztas E, Guven A, Turk E, . 3-Aminobenzamide, a poly ADP ribose polymerase inhibitor, attenuates renal ischemia/reperfusion injury. Ren Fail. 2009;31:393–399.
- Yanarates O, Guven A, Sizlan A, . Ameliorative effects of proanthocyanidin on renal ischemia/reperfusion injury. Ren Fail. 2008;30:931–938.
- Muller MM, Curtius HC, Herold M, . Neopterin in clinical practice. Clin Chim Acta. 1991;201:1–16.
- Ruokonen E, Ilkka L, Niskanen M, . Procalcitonin and neopterin as indicators of infection in critically ill patients. Acta Anaesthesiol Scand. 2002;46:398–404.
- Cayci T, Akgul EO, Kurt YG, . Cord blood and maternal serum neopterin concentrations in patients with pre-eclampsia. Clin Chem Lab Med. 2010;48:1127–1131.
- Gul H, Uysal B, Cakir E, . The protective effects of ozone therapy in a rat model of acetaminophen-induced liver injury. Environ Toxicol Pharmacol. 2012;34:81–86.
- Yaman H, Cakir E, Ozcan O, . Elevated urine neopterin levels in nonalcoholic steatohepatitis. Clin Biochem. 2005;38: 187–190.
- Bocci V. Ozone as Janus: This controversial gas can be either toxic or medically useful. Mediators Inflamm. 2004;13:3–11.
- Bocci VA. Scientific and medical aspects of ozone therapy: State of the art. Arch Med Res. 2006;37:425–435.
- Oter S, Korkmaz A. Relevance of hyperbaric oxygen to ozone therapy. Arch Med Res. 2006;37:917–918.
- Re L, Mawsouf MN, Menendez S, . Ozone therapy: Clinical and basic evidence of its therapeutic potential. Arch Med Res. 2008;39:17–26.
- Bocci V. Does ozone therapy normalize the cellular redox balance? Implications for therapy of human immunodeficiency virus infection and several other diseases. Med Hypotheses. 1996;46:150–154.
- Chatterjee PK, Patel NS, Kvale EO, . Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002;61:862–871.
- Altinel O, Demirbas S, Cakir E, . Comparison of hyperbaric oxygen and medical ozone therapies in a rat model of experimental distal colitis. Scand J Clin Lab Invest. 2011;71:185–192.
- Tunc T, Uysal B, Atabek C, . Erdosteine and ebselen as useful agents in intestinal ischemia/reperfusion injury. J Surg Res. 2009;155:210–216.
- Alrashed M, Abougoush M, Akgul E, . Detection method of serum and urine neopterin levels by high performance liquid chromatography and clinical applications. Gulhane MJ. 2002;44: 273–277.
- Cakir E, Akgul OE, Aydin I, . The association between neopterin and acetaminophen-induced nephrotoxicity. Ren Fail. 2010;32:740–746.
- Rabb H, Ramirez G, Saba SR, . Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol. 1996;271:F408–F413.
- Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett. 2000;486:10–13.
- Zamora ZB, Borrego A, Lopez OY, . Effects of ozone oxidative preconditioning on TNF-alpha release and antioxidant–prooxidant intracellular balance in mice during endotoxic shock. Mediators Inflamm. 2005;1:16–22.
- Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–1134.
- Ajamieh H, Merino N, Candelario-Jalil E, . Similar protective effect of ischemic and ozone oxidative preconditionings in liver ischemia/reperfusion injury. Pharmacol Res. 2002;45: 333–339.
- Chen H, Xing B, Liu X, . Similarities between ozone oxidative preconditioning and ischemic preconditioning in renal ischemia/reperfusion injury. Arch Med Res. 2008; 39:169–178.
- Madej P, Plewka A, Madej JA, . Ozonotherapy in an induced septic shock I: Effect of ozonotherapy on rat organs in evaluation of free radical reactions and selected enzymatic systems. Inflammation. 2007;30:52–58.
- Bocci V, Zanardi I, Travagli V. Has oxygen-ozonetherapy a future in medicine? J Exp Integr Med. 2011;1:5–11.
- Fuchs D, Weiss G, Wachter H. Neopterin, biochemistry and clinical use as a marker for cellular immune reactions. Int Arch Allergy Immunol. 1993;101:1–6.
- Bocci V, Borrelli E, Travagli V, . The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med Res Rev. 2009;29:646–682.
- Caliskan B, Guven A, Ozler M, . Ozone therapy prevents renal inflammation and fibrosis in a rat model of acute pyelonephritis. Scand J Clin Lab Invest. 2011;71:473–480.
- Demirbag S, Uysal B, Guven A, . Effects of medical ozone therapy on acetaminophen-induced nephrotoxicity in rats. Ren Fail. 2010;32:493–497.
- Guven A, Gundogdu G, Sadir S, . The efficacy of ozone therapy in experimental caustic esophageal burn. J Pediatr Surg. 2008;43:1679–1684.
- Guven A, Gundogdu G, Vurucu S, . Medical ozone therapy reduces oxidative stress and intestinal damage in an experimental model of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2009;44:1730–1735.
- Uysal B, Yasar M, Ersoz N, . Efficacy of hyperbaric oxygen therapy and medical ozone therapy in experimental acute necrotizing pancreatitis. Pancreas. 2010;39:9–15.