Abstract
Posterior reversible encephalopathy syndrome (PRES) is a clinicoradiographic entity of heterogenous etiologies, which are grouped together because of similar findings on neuroimaging studies, associated with similar symptom complex of headache, vision loss, altered mentation, and seizures. In this report, we describe a case of PRES in setting of postobstructive diuresis in a 5-year-old male child, whose solitary functioning kidney was obstructed by a 1.6-cm radio-opaque stone, who after percutaneous nephrostomy (PCN) diversion developed persistent hypocalcemia which persisted despite maximum replacement by iv calcium gluconate drip, and the child developed repeated generalized tonic clonic convulsions and became unconscious for 4 days. Computerized tomography (CT) scan of the brain showed typical hypodensities in bilateral occipitoparietal regions suggesting PRES. Ultimately, over a period of 4 days, his hypocalcemia could be corrected and the child was neurologically normal on the 5th day. CT scan of the brain after a month was free of any hypodensities.
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) was first reported as a reversible posterior leukoencephalopathy syndrome (RPLS) in 1996.Citation1 Initially, it was described in acutely ill patients, who had a reversible syndrome of headache, altered mental function, seizure, and loss of vision, associated with findings indicating predominantly posterior leukoencephalopathy on imaging studies.Citation1 Literature consist of few case reports or few small series of patients in different clinical settings.Citation1–3 All age groups are susceptible to this syndrome. The setting in which it was described included patients who were receiving immunosuppressive medicines after organ transplantation or other malignancies, patients with renal failure, patients with hypertensive encephathalopathies, and patients with eclampsia. Other comorbid conditions described later included children with nephrotic syndrome, acute nephritis, hemolytic uremic syndrome, systemic lupus erythematosus, and patients with volume and electrolyte disturbances. Pathogenetic mechanism is believed to be vasogenic edema of cerebral white matter affecting commonly occipitoparietal region due to autoregulatory failure or endothelial dysfunction leading to hyperperfusion and breakdown of blood–brain barrier allowing extravasation of fluid in brain parenchyma.Citation1,4 Predilection for posterior brain regions could be because pial and intracerebral vessels in anterior circulation have higher concentration of adrenergic nerves as compared to posterior hemisphere.Citation5 Diagnosis is made in appropriate clinical setting with neurological symptoms supported by neurological imaging by CT scan, MRI, and DWI (Diffusion Weighted Image).Citation4 Clinicians should have high index of suspicion in the event of neurological symptom complex in at risk clinical settings of PRES and neuroimages should be promptly obtained because most of the time it is reversible if treated early. In this report, we present a first case of child with PRES in setting of postobstructive diuresis and persistent hypocalcemia which reverted completely after treatment.
CASE REPORT
A 5-year-old male child weighing 14 kg was admitted with chief complaints of oliguria, right flank pain, fever, and breathlessness of 4 days duration. On examination, the child was moderately malnourished and was severely pale with heart rate of 112 bpm, respiratory rate of 40 breaths per minute, blood pressure (BP)130/86 mmHg with mild edema face but no peripheral edema and no ascites. His neck veins were engorged and respiratory system on auscultation was found to have bilateral wheezing all over the chest with basal crepitations. His chest X-ray showed bilateral fluffy opacities. His urine showed +1 albumin and was loaded with pus cells. His Hb was 5.4 gm/dL, WBC 11,560/cmm, platelets 667,000/cmm, random blood sugar 96 mg%, blood urea 170 mg/dL, serum creatinine 6.4 mg/dL, sodium 141 mEq/L, chloride 96 mEq/L, potassium 5.4 mEq/L, total calcium 8.8 mg/dL, serum albumin 2.5 gm/dL, and iPTH 540 pg/mL. Blood gas studies on venous sample showed pH 7.2, HCO3 8.5 mmol/L, ionized calcium 0.717 mmol/L, and base deficit of 17.9 mmol/L. Abdomen ultrasound test showed moderate hydronephrosis on right side measuring 10.6 cm × 4.7 cm with renal pelvic stone 1.6 cm. Left kidney was 10.4 cm × 4 cm with gross hydronephrosis and paper thin parenchyma with internal echoes and debris suggesting pyonephrosis with ureteropelvic junction obstruction. Urine culture showed Escherichia coli sensitive to levofloxacin and cefoperazone–sulbactam combination. ELISA resusts for HBSAg, HIV, and HCV were negative.
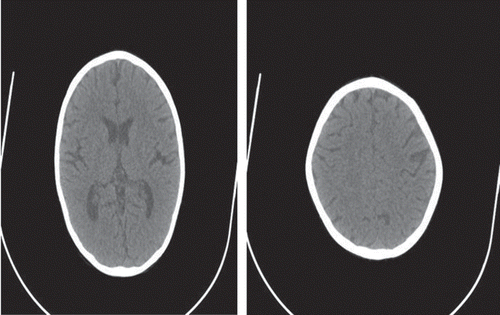
The child was started having Nifedipine retard tablet of 10 mg twice a day and was treated with iv cefoperazone–sulbactam 750 mg BD twice a day. He was offered acute peritoneal dialysis (PD) for 24 hours and 30 cycles of PD with 30 minutes dwell time were completed. The child was given 30 mL sodium bicarbonate iv in drip in 0.45 N Saline. The child was also given 5 mL calcium gluconate diluted iv for every 6 hour during the PD. The child received 150 mL of packed cell volume and 100 mL 20% human albumin also during the PD. The child was posted for bilateral percutaneous nephrostomy (PCN) after the PD was over when preoperatively his BP was 110/70 mmHg and Hb was 8.0 gm/dL. Venous blood gas study showed pH 7.3, HCO3 20.8 mmol/L, Na 138 mmol/L, chloride 100 mmol/L, K 3.96 mmol/L, Ca 0.944 mmol/L and X-ray of the chest was normal. Bilateral PCN was done by 10 French PCN tube. His right PCN drained 350 mL in first 6 hours whereas left PCN tube drained 30 mL initially and had no increase of drain in 24 hours. Postoperatively child’s BP was 140/90 mmHg. He was receiving 10 mL less iv fluid than total PCN drain for every 4 hours. The child was given Nifedipine tablet of 5 mg repeatedly whenever his BP rose above 120/80 mmHg. After 6 hour of PCN drainage, the child had first attack of generalized tonic clonic convulsion, which was treated by 5 mg diluted iv diazepam and sample for electrolytes and blood gas was taken which showed pH 7.32, HCO3 17.5 mmol/L, Na 148 mEq/L, K 2.89 mEq/L, Cl 107 mEq/L, ionized Ca 0.515 mmol/L, and Mg 1.77 mg/dL. His blood urea was 97 mg/dL, serum creatinine 4.1 mg/dL, and total Ca 5.8 mg/dL. The child was given 5 mL of calcium gluconate stat and started with calcium gluconate drip in one iv line and sodium bicarbonate drip in another iv line. The child had repeated convulsions at interval of 1–2 hours for 12 hours for which he was loaded with phenytoin 200 mg drip over 2 hour and he was maintained on phenytoin 50 mg infusion for 8 hours till seizure was controlled. He was given magnesium sulfate half milliliter intramuscular (i.e., 250 mg–25% w/v). His venous blood gas with electrolytes was repeated for every 6 hours. The child received 25 mL of calcium gluconate and 20 mL of sodium bicarbonate in the first 24 hours. His right PCN drained 650 mL in 24 hours and left PCN had total drain of 40 mL. His fundus examination was normal. The next day morning, plain CT scan of the brain was performed, which showed bilateral hypodensities in occipitoparietal region suggesting PRES (). At the same time, the child’s creatinine level was 3.8 mg%, Na 142 mmol/L, K 3.6 mmol/L, total Ca 6.5 mg/dL, and PO4 5.4 mg/dL. His venous blood gas showed pH 7.36, HCO3 18 mEq, Na 141 mmol/L, chloride 104 mmol/L, K 3.4 mmol/L, and ionized Ca 0.612 mmol/L. The child was continued on calcium gluconate drip and sodium bicarbonate drip in stepwise decreasing dose according to venous blood gas and ionized calcium reports for 4 days. Seeing persistent hypocalcemia, we measured 24-hour urinary Ca, which was 30 mg/24 hour (normal range 42–350 mg).
Figure 1. Diffuse cerebral edema and ill-defined hypodensities in subcortical white matter at bilateral parieto-occipital and frontal regions.
Frequency of convulsion was decreasing from next day. Urine output in right PCN was also increasing day by day, whereas left PCN had only little milliliter pus drained. Convulsions were controlled after 4 days. The child was conscious on the 5th day without any sensorimotor deficit. His serum creatinine on 5th day was 2.28 mg/dL, Na 138 mmol/L, K 3.6 mmol/L, chloride 102 mmol/L, total Ca 9.2 mg/dL, PO4 4.8 mg/dL, pH 7.38, HCO3 20.8 mEq/L, ionized Ca 0.88 mmol/L, and iPTH 408 pg/mL. From 5th day onward, the child was started having Nifedipine retard tablet of 10 mg twice a day, syrup calcium carbonate 15 mL, 8 hourly, tablet compound sodium bicarbonate 250 mg three tablets three times a day which required to be increased to three tablets (i.e., 750 mg) four times a day after 4 days to maintain his bicarbonate above 20 mEq/L, cap 1 alpha calcidiol (0.25 μg) once a day and Phenytoin tablet of 100 mg twice a day.
Twelve days later, the child was posted for percutaneous nephrolithotomy on right side with DJ stenting when his Hb was 8.5 gm, serum creatinine 2.2 mg/dL, Na 132 mmol/L, K 4.6 mmol/L, Ca 8.8 mg%, and HCO3 20 mEq/L.
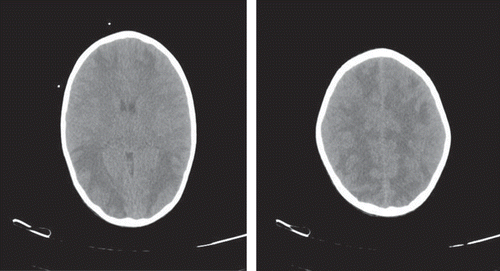
One month later, right DJ stent was removed when his serum creatinine was 1.9 mg%, K 4.5 mmol/L, Na 140, and Ca 8.9 mgdL, HCO3 level 19 mEq/L, and BP 110/70 mmHg with Nifedipine retard tablet of 10 mg twice a day. His left PCN was draining 100 mL urine after a month against 1200 mL urine output per urethra. His repeat CT scan of the brain after a month showed total disappearance of hypodensities from cerebral hemispheres (). Now the Child is planned for left-sided nephrectomy.
DISCUSSION
Clinical settings in which PRES is known to occur and its pathophysiologic mechanism is already described in the “Introduction” section. Because of the heterogeneous settings of this disorder, it may be that different mechanisms are involved in different clinical settings or more than one contributory factor could be operating in an individual setting.
PRES in setting of postobstructive diuresis and persistent hypocalcemia is described for the first time in the present case. The child, in addition, persisted to have below normal renal function and had associated hypertension postoperatively which was controlled with medication. The child’s metabolic status and electrolyte balance except for hypocalcemia were always better postoperatively than previously when he presented with renal failure and obstructed solitary functioning kidney. He was remaining mildly acidotic despite adequate alkali replacement because of wasting of bicarbonate due to failure of proximal tubular reabsorption and associated defect in distal tubule also. Persistence of hypocalcaemia despite adequate calcium replacement over few days could be associated with defect in proximal tubular reabsorption of sodium and water which prevented facilitated absorption of calcium from proximal tubules. Excess bicarbonate in the tubular fluid also prevented calcium absorption from distal tubule.Citation6 Persistent hyperparathyroidism also prevented calcium absorption from proximal tubules. Twenty-four hour urinary calcium was not very high in absolute terms, but it was relatively high in face of very low serum ionized calcium levels. Secondly, patient’s iPTH has improved from 540 pg/mL preoperatively to 408 pg/mL after 5 days of diversion. This improvement in iPTH could be because of the improvement of reversible element of acute renal failure. This fall in iPTH could have stimulated Ca uptake of bone leading to persistent hypocalcemia, despite the continuous iv replacement of calcium gluconate. All these are logical explanations for hypocalcemia in this setting because we have observed this patient of its kind first time. But documented occurrence of PRES in association with persistent hypocalcemia and reversal of neurosyndrome and neuroimages after its correction points toward causal relationship. Calcium has unique intracellular messenger role and it is complexed with numerous ligands in cytosol including adenosine, vitamin D–dependent, calcium-binding protein calmodulin.Citation6 Persistent hypocalcemia may be expected to affect directly or indirectly mechanisms involved in autoregulation and endothelial function.
CONCLUSION
We are still a long way in understanding PRES in general and persistent hypocalcemia in particular in setting of postobstructive diuresis. A better comprehensive metabolic monitoring of postobstructive diuretic patients can allow prompt and appropriate correction of existing metabolic disorder. Persistent hypocalcemia is one of the metabolic disorders found in postobstructive diuresis, which is associated with neurologic syndrome like PRES.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Hinchey J, Chaves C, Appignani B, . A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494.
- Kwon S, Koo J, Lee S Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol. 2001;24:361.
- Ishikura K, Ikeda M, Hamasaki Y, . Nephrotic state as a risk factor for developing posterior reversible encephalopathy syndrome in paediatric patients with nephrotic syndrome. Nephrol Dial Transplant. 2008;23:2531–2536.
- Schwartz RB, Mulkern RV, Gudbjartsson H, Jolesz F. Diffusion-weighted MR imaging in hypertensive encephalopathy: Clues to pathogenesis. Am J Neuroradiol. 1998;19:859–862.
- Beausang-Linder M, Bill A Cerebral circulation in acute arterial hypertension – protective effects of sympathetic nervous activity. Acta Physiol Scand. 1981;111:193.
- Sutton RAL, Dirks JH. Calcium and magnesium: Renal handling and disorders of metabolism. In: Brenner BM, Rector FC Jr, eds. The Kidney. Philadelphia, PA: WB Saunders; 1986:551–618.