Abstract
Objectives: Cardiovascular risk is increased in the early stages of chronic kidney disease (CKD) and is also found to be ongoing in renal transplant (Rtx) patients. As a sign of atherosclerosis, increased carotid intima–media thickness (CIMT) has been widely accepted as a strong predictor of cardiovascular disease (CVD) and mortality in the end-stage renal disease (ESRD) patients. Ischemia-modified albumin (IMA), pentraxin-3 (PTX-3), and neutrophil-to-lymphocyte ratio (NLR) were introduced as oxidative stress and inflammatory biomarkers in ESRD. The role of Rtx in terms of atherogenesis, oxidative stress, and inflammation is still unclear. We aimed to investigate the relationship between IMA, PTX-3, NLR, and CIMT in Rtx patients without overt CVD and to compare these results with those obtained from healthy subjects and ESRD patients receiving hemodialysis (HD) and peritoneal dialysis (PD). Design and methods: Cross-sectional analysis in which CIMT measurements, NLR, and serum PTX-3 and IMA levels were assessed in 18 Rtx patients (10 females; mean age: 40.0 ± 13.3 years), 16 PD patients (7 females; 40.2 ± 12.9 years), 14 HD patients (8 females; 46.6 ± 10.7 years), and 19 healthy subjects (9 females; 36.9 ± 8.9 years). Results: IMA, PTX-3, and high-sensitive C-reactive protein (hs-CRP) levels, NLR, and CIMT of Rtx patients were found to be significantly higher compared with healthy subjects ( p = 0.04, p < 0.0001, p < 0.005, p = 0.005, and p = 0.005, respectively). IMA level was positively correlated with hs-CRP and PTX-3 levels, NLR, and CIMT when all participants were included (r = 0.338, p = 0.005; r = 0.485, p < 0.0001; r = 0.304, p = 0.013; and r = 0.499, p < 0.0001, respectively). Conclusion: There has been ongoing inflammation, oxidative stress, and atherosclerosis in Rtx patients.
INTRODUCTION
Cardiovascular diseases (CVDs) are still the leader of the etiology of morbidity and mortality in patients with chronic kidney disease (CKD).Citation1 Besides traditional risk factors, many nontraditional novel risk factors including inflammation, endothelial dysfunction, vascular calcification, and oxidative stress were identified to define these heightened death rates in this population.Citation2,3 Several studies demonstrated that systemic persistent inflammation could be the main factor responsible for this increased death risk in end-stage renal disease (ESRD) patients regardless of the renal replacement therapy (RRT).Citation4 Several biomarkers including C-reactive protein (CRP), interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) were studied to assess relation of inflammation and CVD in general and CKD populations.Citation4,5 As a sign of atherosclerosis, increased intima–media thickness (IMT) of common carotid artery has been widely used and accepted as a strong predictor of cardiovascular events and mortality in ESRD patients.Citation6 White blood cell (WBC) count and its subtypes were also found to be markers of inflammation in CVD.Citation7 Recently, neutrophil-to-lymphocyte ratio (NLR) was introduced as a potential marker to determine inflammation in cardiac and noncardiac disorders.Citation8–10 NLR was also shown as a predictor of long-term mortality in patients who underwent percutaneous coronary intervention.Citation11 In addition, we recently demonstrated that the NLR could predict inflammation in ESRD patients.Citation12
Pentraxin-3 (PTX-3) is a novel molecule, which has been found to be closely associated with the pathogenesis of atherosclerosis.Citation13 PTX-3 is produced by various cells including monocytes/macrophages, neutrophils, vascular smooth muscle cells, fibroblasts, and endothelial cells.Citation14 Recent studies have shown that PTX-3 is elevated in CKD and associated with endothelial dysfunction and mortality in this population.Citation15,16 However, there are conflicting results about the exact role of PTX-3 in atherosclerotic vascular disease. Some investigators concluded that PTX-3 has atheroprotective effects,Citation17,18 whereas others suggested that it could have atherogenetic properties.Citation13,15
Uremic patients are also faced with abundant oxidative stress, before and after RRT, evidenced by higher concentration of oxidative stress biomarkers including oxidized low-density lipoprotein (LDL), nitrotyrosine, advanced oxidation protein products (AOPPs), and advanced glycation end products (AGEs).Citation3 Recently, ischemia-modified albumin (IMA), a novel ischemia-associated biomarker, was also introduced to assess oxidative stress in CKD, ESRD, obese, and dyslipidemic patients.Citation19–22 Sharma et al.Citation23 also demonstrated that IMA could predict mortality in ESRD patients awaiting renal transplantation (Rtx).
The role of Rtx in terms of oxidative stress, inflammation, and atherogenesis is still unclear. To date, in the literature, there has been no study investigating the relationship between atherosclerosis, IMA, NLR, and PTX-3 in Rtx patients with well-functioning kidney. Hence, we aimed to investigate the relationship between IMA, PTX-3, NLR, and carotid IMT (CIMT) in Rtx patients and to compare these results with those obtained from healthy subjects and hemodialysis (HD) and peritoneal dialysis (PD) patients.
PATIENTS AND METHODS
The study protocol was approved by the Medical Ethics Committee of Selcuk University (Meram School of Medicine, Konya, Turkey). Written informed consent was obtained from all subjects included in the study.
This was a cross-sectional study involving 18 Rtx patients (10 females, 8 males; mean age: 40.0 ± 13.3 years), 16 PD patients (7 females, 9 males; 40.2 ± 12.9 years), and 14 HD patients (8 females, 6 males; 46.6 ± 10.7 years) followed at least 6 months in the transplantation and dialysis units of Necmettin Erbakan University and 19 healthy subjects (9 females, 10 males, mean age: 36.9 ± 8.9 years) between January and November 2011. All patients enrolled in the study were randomly assigned. Rtx patients received their grafts and dialysis patients received HD or PD treatments at least 6 months prior to the study. Mean transplant duration of Rtx patients was 47.3 ± 33.6 months.
All Rtx patients were operated by the same general surgery team in the Rtx unit of Necmettin Erbakan University. Patients aged 18–70 years willing to participate in the assessment of CIMT by carotid duplex ultrasonography were screened. A review of medical records (including information on age, sex, weight, medications, and primary disease of ESRD) was undertaken. Exclusion criteria were (1) angina pectoris and/or documented coronary artery disease, (2) congestive heart failure, (3) active infection, (4) autoimmune disease, (5) severe secondary hyperparathyroidism (patients with intact parathormone (iPTH) >500 pg/mL), and (6) nephrotic-range proteinuria. Forty-three Rtx patients were screened and 25 patients were excluded from the study. Of 25 patients, 9 had documented coronary artery disease, 6 had congestive heart failure (New York Heart Association (NYHA) class III–IV), 5 had active infection, and 5 had severe secondary hyperparathyroidism. None of the patients included in the study had nephrotic-range proteinuria and arrhythmia based on electrocardiography (ECG). The remaining 18 Rtx patients that fulfilled the above criteria were enrolled in the study. Sixteen PD and 14 HD age- and sex-matched ESRD patients and 19 normotensive healthy individuals that were referred from outpatient clinics of the Internal Medicine Department of Necmettin Erbakan University were enrolled as control subjects. They were subjected to the same inclusion and exclusion criteria as the patients.
The systolic blood pressure (SBP) and diastolic blood pressure (DBP) of patients and healthy subjects were measured in the upright sitting position after ≥5 min of rest using an Erka sphygmomanometer (PMS Instruments Limited, Berkshire, UK) with an appropriate cuff size. Two readings were recorded for each individual. The mean value of two readings was defined as the blood pressure. Patients with SBP ≥140 mm Hg and DBP ≥90 mm Hg were assumed to be hypertensive.
Fourteen Rtx patients were taking antihypertensive drugs (all of them were on verapamil therapy). Two patients were taking oral calcium–vitamin D combination, and four patients were on active vitamin D. As the maintenance immunosuppressive therapy, of 18 Rtx patients, 18 (100%) were on prednisolone, 12 (66.6%) were on tacrolimus, 18 (100%) were on mycophenolate mofetil (MMF), 3 (16.7%) were on cyclosporine-A (Cyc-A), and 3 (16.7%) were on everolimus therapy. Four patients (22.2%) were on intensive insulin therapy and one (5.6%) was on oral anti-diabetic medication. Donor source was living-related in nine (50%), living-unrelated in one (5.5%), and deceased donor in eight (44.5%) patients. None of the patients had transplant nephrectomy.
Biochemical Analyses
Venous blood samples were drawn after an overnight fast and stored at −80°C for biochemical analyses in healthy subjects and Rtx patients, and before the first exchange in PD patients and before the mid-week session in patients receiving HD.
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities and uric acid, creatinine, calcium, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), phosphorus (P), urinary protein, and creatinine levels were analyzed on Synchron LX20 system (Beckman Coulter, USA) with the original Beckman reagents. HDL-C levels were determined using direct enzymatic method without precipitation on Synchron LX20 system (Beckman Coulter). LDL-C levels were calculated by Friedewald formula.Citation24 Serum high-sensitive C-reactive protein (hs-CRP) levels were measured with a high-sensitive immunoturbidimetric assay (Diasis Diagnostic System, Beckman Coulter, Fullerton, CA) using an automated clinical chemistry analyzer. Normal range reference interval of hs-CRP for adults was accepted as <10 mg/dL.
Plasma IMA Measurements
We used a manual colorimetric assay described by Bar-Or et al.Citation25 to access the ability of binding exogenous cobalt (Co(II)) to human albumin in serum. A 50 μL water solution of 0.1% cobalt chloride (CoCl26H2O) was added to 200 μL of plasma and gently mixed, and after 10 min (for adequate Co(II) albumin binding), the 50 μL of dithiothreitiol (DTT) solution (1.5 mg/mL H2O) was added as a colorizing agent and the reaction was quenched 2 min later by adding 1.0 mL of 0.9% NaCl. Color development with DTT was measured spectrophotometrically at 470 nm in comparison with a plasma Co(II) blank without DTT and reported in absorbance units (ABSU).
Plasma PTX-3 Measurements
In Rtx, HD, and PD patients and healthy controls, plasma PTX-3 levels were measured by a commercially available kit based on quantitative sandwich enzyme immunoassay technique (R&D Systems, Human Pentraxin 3/TSG-14 Immunoassay kit, Cat no: DPTX3, Minneapolis, MN).
Definition of NLR
Complete blood counts with automated differential counts, which included total WBC, neutrophils, and lymphocytes, were obtained at the time of admission. NLR was calculated as the ratio of the neutrophils and lymphocytes, both obtained from the same automated blood sample at the admission of the study.
GFR Assessment
Glomerular filtration rate (GFR) was calculated according to the simplified version of the Modification of Diet in Renal Disease (MDRD) Study prediction equation formula: GFR = 186 × creatinine−1.154 × age−0.203 × 1.212 (if African-American) × 0.742 (if female), as defined by Levey et al.Citation26
CIMT Measurements
The CIMT recordings were performed by a single investigator (OO), who was blinded for all groups. The carotid arteries were evaluated with the Vivid 7 echocardiography device (General Electrics, Horten, Norway) by using a 10-MHz linear probe. The acquired images were recorded for playback analysis and were later measured off-line. The common carotid artery, the carotid bulb, and the internal and external carotid arteries were visualized on both sides. The IMT of the carotid arteries were measured in the distal common carotid artery at a level of 15–20 mm proximal to the carotid bulb. The two bright echogenic lines in the arterial wall were identified as the intima and the media. Three measurements were made for each side of the body; separate means were calculated and recorded as the right and left IMT. The intra-observer coefficient of variation for CIMT was 2.5%.
Statistical Analyses
Statistical analyses were carried out using the Statistical Package for Social Sciences for Windows version 15.0 (SPSS, Chicago, IL, USA). Data were expressed as the mean ± SD, with a significance level of p < 0.05. The normal distributions of all variables were tested using the Kolmogorov–Smirnov test. Dichotomous variables were compared using the chi-square test. Statistical differences between parametric data of four groups were analyzed using the analysis of variance (ANOVA) test. The Kruskal–Wallis test was used to determine differences between nonparametric data. The nonparametric Spearman coefficient of correlation was used to assess correlations between variables without normal distribution.
RESULTS
Patients’ Baseline Characteristics
The baseline characteristics of Rtx, HD, and PD patients and healthy subjects were depicted in . The etiologies of renal disease of the Rtx patients were chronic glomerulonephritis (n = 4, 22.2%), diabetic nephropathy (n = 4, 22.2%), hypertensive nephropathy (n = 3, 16.6%), polycystic kidney disease (n = 1, 5.6%), and unknown etiology (n = 6, 33.3%). Of 18 Rtx patients, 8 (44.4%) had posttransplant hypertension, 6 (33.3%) had posttransplant dyslipidemia, and 4 (22.2%) had new-onset diabetes after transplantation (NODAT). There were no differences in age, gender, body mass index (BMI), biochemical parameters including serum LDL-C, ALT, P, and hemoglobin levels between Rtx, HD, and PD patients and healthy subjects. Spot urine protein-to-creatinine ratio (PCR) values of healthy controls and Rtx patients were 0.09 ± 0.09 (g/mg) and 0.4 ± 0.5 (g/mg), respectively (p < 0.0001). Serum creatinine, uric acid, TG, and calcium levels were significantly higher in Rtx patients compared with healthy controls (p < 0.0001, p < 0.0001, p = 0.001, and p = 0.0001, respectively). Estimated GFR measurements were also found to be significantly lower in Rtx patients than in healthy subjects (56 ± 27 mL/min vs. 121 ± 28 mL/min, respectively, p < 0.0001).
Table 1. The demographic, clinical, and laboratory features of the healthy subjects and Rtx, HD, and PD patients.
IMA, PTX-3, NLR, hs-CRP, and CIMT Measurements
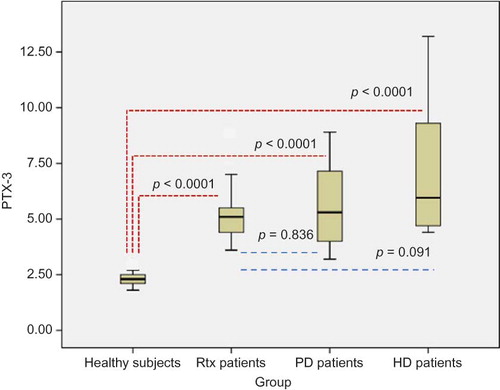
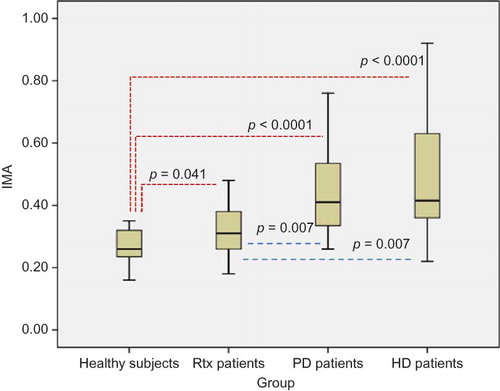
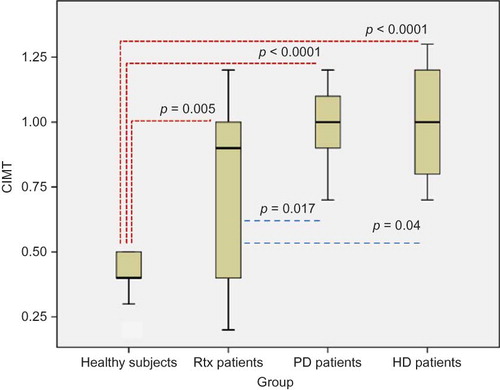
Results of oxidative stress, inflammation, and atherosclerosis markers including IMA, PTX-3, hs-CRP, NLR, and CIMT were shown in . Serum IMA, PTX-3, and hs-CRP levels of Rtx patients were found to be significantly higher compared with healthy subjects (p = 0.041, p < 0.0001, and p < 0.005, respectively). Neutrophil counts and NLR were also significantly higher in Rtx patients compared with healthy controls (p < 0.0001, 0.005, respectively) (). The mean CIMT of Rtx patients and healthy subjects was 0.7 ± 0.3 mm and 0.4 ± 0.08 mm, respectively (p = 0.005) (). Serum IMA, PTX-3, and hs-CRP levels, NLR, and CIMT of healthy subjects were found to be significantly lower compared with PD and HD patients (p < 0.0001, for all) (). Rtx patients had significantly lower serum IMA than both PD and HD patients (p = 0.007, for both) (). There were no statistically significant differences between Rtx patients and HD and PD patients in terms of PTX-3 and hs-CRP levels and NLR ( and ). Rtx patients had significantly lower CIMT compared with both HD and PD patients (p = 0.04 and p = 0.017, respectively) ().
Figure 1. NLR values of healthy subjects and Rtx, PD, and HD patients. NLR, neutrophil-to-lymphocyte ratio; Rtx, renal transplant; PD, peritoneal dialysis; HD, hemodialysis.
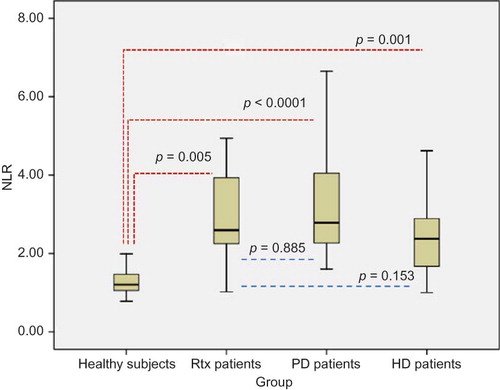
Figure 2. CIMT of healthy subjects and Rtx, PD, and HD patients. CIMT, carotid intima–media thickness; Rtx, renal transplant; PD, peritoneal dialysis; HD, hemodialysis.
Univariate Correlations between IMA, NLR, PTX-3, CIMT, and Continuous Variables
To investigate the relationship between oxidative stress and inflammation, we performed a univariate correlation analysis. IMA level was positively correlated with hs-CRP and PTX-3 levels and NLR when all participants of groups were included (r = 0.338, p = 0.005; r = 0.485, p < 0.0001; and r = 0.304, p = 0.013, respectively) (). IMA was also significantly correlated with CIMT (r = 0.499, p < 0.0001) (). IMA and PTX-3 levels, NLR, and CIMT were also positively correlated with serum creatinine, P, uric acid, TG, and spot urine PCR levels and negatively correlated with estimated glomerular filtration rate (eGFR) and serum albumin levels when all participants of the groups were studied ().
Table 2. Univariate correlations of IMA, CIMT, and PTX-3 with continuous variables.
DISCUSSION
This cross-sectional study mainly showed that oxidative stress measured by serum IMA levels, inflammation measured by serum PTX-3 and hs-CRP levels and NLR, and atherosclerosis defined by CIMT persisted in Rtx patients despite a significant improvement in kidney function compared with ESRD patients receiving HD and PD. To the best of our knowledge, this is the first study assessing the role of IMA as a marker of oxidative stress in Rtx and its association with inflammation and atherosclerosis.
Contemporary research regarding nontraditional risk factors revealed that oxidative stress is an important cardiovascular risk factor in uremic patients.Citation27 Despite the improvement of kidney function in Rtx patients, lower eGFR places them in one of the stages of CKD. Hence, Rtx patients might be considered as a subset of CKD patients. Recently, IMA was introduced as a novel oxidative stress biomarker in uremic patients.Citation19,20 Moreover, Sharma et al.Citation23 demonstrated that serum IMA levels predict mortality in ESRD patients. They also showed that higher IMA levels were found to be associated with increased left ventricular (LV) size and filling pressures and decreased LV systolic function in this population. In this study, we found that HD and PD patients had higher serum IMA levels compared with Rtx patients and healthy subjects. Additionally, IMA levels were found to be significantly higher in Rtx patients compared with healthy controls. This means that there has been an ongoing oxidative stress in Rtx patients despite a well-functioning kidney.
PTX-3, a member of long PTXs, acts as an immunomodulator involving in humoral arm of immunityCitation28 and also participates in inflammation, angiogenesis, and CVD.Citation29 Growing evidence suggests that PTX-3 is one of the most rapid inflammatory markers circulating in the early phase of inflammation milieu.Citation30 Tong et al.Citation31 showed that PTX-3 levels were increased in CKD patients, and according to this study, CKD patients with high PTX-3 levels had higher cardiovascular mortality. The same group also demonstrated that PTX-3 and proteinuria were independently associated with endothelial dysfunction in type 2 diabetic patients.Citation15 Moreover, short-term blockage of renin–angiotensin–aldosterone system with angiotensin-converting enzyme (ACE) inhibitor treatment improves endothelial function, diminishes proteinuria, and normalizes PTX-3 and hs-CRP.Citation32 In this study, we assessed inflammation parameters including serum hs-CRP and PTX-3 levels in Rtx, HD, and PD patients, and healthy subjects. Rtx patients had higher serum PTX-3 than healthy subjects; however, they had lower levels of this parameter compared with HD and PD patients. The levels of hs-CRP were found to be significantly higher in Rtx patients compared with healthy subjects. Nonetheless, there were no statistically significant differences between Rtx, HD, and PD patients in terms of hs-CRP. We also showed that PTX-3 levels were positively correlated with CIMT in all subjects. However, we failed to demonstrate any correlation between PTX-3 and CIMT in Rtx patients (data not shown). There has been a debate regarding the exact role of PTX-3 in inflammation and atherosclerosis. Some researchers suggested that PTX-3 might have a cardioprotective role as shown in a mouse model of acute myocardial infarction.Citation17 According to the results of this study, in wild-type mice, PTX-3 levels were increased after the onset of acute myocardial injury (AMI); however, PTX-3-deficient mice showed more infarction area of myocardium, which was associated with increased neutrophil infiltration and serum IL-6 levels, apoptosis of cardiomyocytes, and no reflow area in the heart. In addition, to prove the cardioprotective effect of PTX-3, PTX-3-deficient and wild-type mice were treated with human recombinant PTX-3. They also demonstrated that human recombinant PTX-3 therapy significantly reduced the extent of cardiac damage and IL-6 levels in PTX-3-deficient mice. The expression of PTX-3 was also found to be induced by HDL in human and mice endothelial cells.Citation33 Furthermore, Deban et al.Citation34 reported that PTX-3 could have a role in dampening of excessive neutrophil recruitment, which may lessen inflammation. All these findings were strengthened with the observation that PTX-3 deficiency is also associated with increased atherosclerosis and macrophage infiltration in these atherosclerotic lesions in apolipoprotein-E-deficient mice.Citation18 Currently, the exact role of PTX-3 is unknown in both CKD and Rtx population. On the basis of experimental and clinical data mentioned above, we hypothesized that PTX-3 might had endothelial protective effects rather than deleterious effects in Rtx patients. The main basis of our hypothesis was that atherosclerosis was found to be much more improved in Rtx patients compared with ESRD patients despite the ongoing inflammation in this population. Hence, one might hypothesize that kidney transplantation may not completely reverse nontraditional risk factors including inflammation and oxidative stress. This may partly explain why Rtx patients had high PTX-3 and hs-CRP levels compared with healthy subjects.
Calculation of NLR is a very simple and cheap method when compared with the other inflammatory cytokines including IL-6, IL-1β, and TNF-α. WBC count and its subtypes are known as classic markers of inflammation in CVD.Citation7 Recently, neutrophilia and relative lymphocytopenia were shown to be an independent predictor of mortality in patients with acute heart failure.Citation35,36 Moreover, NLR was introduced as a potential marker to determine inflammation in cardiac and noncardiac disorders.Citation8–10 Additionally, NLR was shown as a predictor of long-term mortality in patients that underwent percutaneous coronary intervention.Citation11 In our previous study, we demonstrated that NLR was positively correlated with TNF-α in ESRD patients.Citation12 To date, in the literature, there has been no study evaluating NLR and its association with oxidative stress in Rtx patients. In this study, NLR was higher in Rtx patients compared with healthy subjects and HD patients; however, NLR was found to be lower in Rtx patients than in PD patients. Mean neutrophil counts were found to be higher in our Rtx patients compared with healthy controls and HD and PD patients. We believe that these increments in neutrophil counts were regardless of using corticosteroid therapy because all of our patients were using low-dose prednisolone (5 mg/day) that might not alter circulating amount of neutrophils. Although Rtx patients had also used lymphocyte-depleting agents as a maintenance immunosuppressive therapy, there were no significant differences between Rtx patients, healthy controls, and ESRD patients in terms of lymphocyte counts. Thus, increased NLR might be related to ongoing inflammation in this population.
Ultrasonographically measured CIMT was found to be a well-validated marker of atherosclerosis, as well as a commonly used surrogate end point in clinical trials.Citation37 Recently, Hornum et al.Citation38 demonstrated a significant improvement in endothelial function, mean arterial pressure, and plasma von Willebrand factor (vWF) levels but not in plasma CRP and albumin levels in ESRD patients that received Rtx. Seyahi et al.Citation39 also showed that Rtx did not reverse or halt coronary artery calcification in this population. CIMT of our Rtx patients were found to be significantly higher compared with healthy controls and significantly lower compared with HD and PD patients. We also demonstrated that CIMT was positively correlated with serum creatinine, P, uric acid, TG, hs-CRP, PTX-3, IMA, and spot urine PCR levels and NLR and negatively correlated with eGFR and serum albumin levels when healthy subjects and ESRD and Rtx patients were included in the model. However, we could not find these correlations when we analyzed the groups separately. Small sample sizes of each group might explain the absence of significance in correlation analyses. Our results are partially in accord with the study investigating the predictors of CIMT in a large CKD cohort.Citation40 In this study, serum hs-CRP, uric acid, P, iPTH, CaXPO4 product, homeostatic model assessment (HOMA)-insulin resistance, and mean arterial pressure levels were found to be positively correlated, whereas eGFR and serum Ca levels were negatively correlated with CIMT in patients with CKD stages 1–5.Citation40 They also showed that CIMT values decreased significantly after 30 and 90 days of Rtx in subgroup analysis.Citation40 Uremic atherosclerosis is associated with the generation of AGEs.Citation41 AGEs may also contribute to oxidative stress and inflammation.Citation42 Thus, by virtue of ongoing inflammation and oxidative stress, our Rtx patients might have more atherosclerotic vessels than controls.
Elevated uric acid levels were found to be associated with the development of hypertension, stroke, diabetes, and heart diseases.Citation43–45 Recently, Kanbay et al.Citation46 also demonstrated that hyperuricemic subjects have worse endothelial function, and the treatment of hyperuricemia with allopurinol improves endothelial dysfunction. In this study, serum uric acid levels were found to be positively correlated with PTX-3 and IMA levels and CIMT. None of our patients have symptomatic gout; however, patients with high uric acid levels also have worse endothelial dysfunction and increased inflammation and oxidative stress markers.
Our study had some limitations. First, the study sample was relatively small. Second, since this was a cross-sectional study, we could not draw a cause-and-effect relationship from our findings.
In conclusion, this study showed that oxidative stress, inflammation, and atherosclerosis persist in Rtx patients without known CVD. Rtx is the preferred replacement therapy in ESRD patients all over the world; however, this option cannot completely cure all metabolic disturbances in this population. Further randomized controlled trials investigating the relationship between atherosclerosis, inflammation, and oxidative stress in Rtx patients are needed.
ACKNOWLEDGMENTS
Dr. A. Gaipov received grant support from the ERA–EDTA fellowship program.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci. 2003;325(4):163–167.
- Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: Results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16(2):529–538.
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5):1524–1538.
- Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008; 3(2):505–521.
- Turkmen K, Gorgulu N, Uysal M, . Fetuin-A, inflammation, and coronary artery calcification in hemodialysis patients. Indian J Nephrol. 2011;21(2):90–94.
- Benedetto FA, Mallamaci F, Tripepi G, Zoccali C. Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol. 2001;12(11): 2458–2464.
- Horne BD, Anderson JL, John JM, . Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643.
- Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102(6):653–657.
- Nunez J, Nunez E, Bodi V, . Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101(6):747–752.
- Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–184.
- Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97(7):993–996.
- Turkmen K, Guney I, Yerlikaya FH, Tonbul HZ. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren Fail. 2012;34(2):155–159.
- Savchenko A, Imamura M, Ohashi R, . Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol. 2008;215(1):48–55.
- Cieslik P, Hrycek A. Long pentraxin 3 (PTX3) in the light of its structure, mechanism of action and clinical implications. Autoimmunity. 2012;45(2):119–128.
- Suliman ME, Yilmaz MI, Carrero JJ, . Novel links between the long pentraxin 3, endothelial dysfunction, and albuminuria in early and advanced chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(4):976–985.
- Boehme M, Kaehne F, Kuehne A, . Pentraxin 3 is elevated in hemodialysis patients and is associated with cardiovascular disease. Nephrol Dial Transplant. 2007;22(8):2224–2229.
- Salio M, Chimenti S, De Angelis N, . Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117(8):1055–1064.
- Norata GD, Marchesi P, Pulakazhi Venu VK, . Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120(8):699–708.
- Turedi S, Cinar O, Yavuz I, . Differences in ischemia-modified albumin levels between end stage renal disease patients and the normal population. J Nephrol. 2010;23(3):335–340.
- Cichota LC, Moresco RN, Duarte MM, da Silva JE. Evaluation of ischemia-modified albumin in anemia associated to chronic kidney disease. J Clin Lab Anal. 2008;22(1):1–5.
- Piva SJ, Duarte MM, Da Cruz IB, . Ischemia-modified albumin as an oxidative stress biomarker in obesity. Clin Biochem. 2011;44(4):345–347.
- Duarte MM, Rocha JB, Moresco RN, . Association between ischemia-modified albumin, lipids and inflammation biomarkers in patients with hypercholesterolemia. Clin Biochem. 2009;42(7–8):666–671.
- Sharma R, Gaze DC, Pellerin D, . Ischemia-modified albumin predicts mortality in ESRD. Am J Kidney Dis. 2006;47(3):493–502.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
- Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt–albumin binding and its potential as a marker for myocardial ischemia—A preliminary report. J Emerg Med. 2000;19(4):311–315.
- Levey AS, Berg RL, Gassman JJ, Hall PM, Walker WG. Creatinine filtration, secretion and excretion during progressive renal disease. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney Int Suppl. 1989;27:S73–S80.
- Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22(6):636–643.
- Bottazzi B, Garlanda C, Cotena A, . The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: Interplay with cellular innate immunity. Immunol Rev. 2009; 227(1):9–18.
- Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23: 337–366.
- Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: A modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med. 2010;20(2):35–40.
- Tong M, Carrero JJ, Qureshi AR, . Plasma pentraxin 3 in patients with chronic kidney disease: Associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol. 2007;2(5):889–897.
- Yilmaz MI, Axelsson J, Sonmez A, . Effect of renin angiotensin system blockade on pentraxin 3 levels in type-2 diabetic patients with proteinuria. Clin J Am Soc Nephrol. 2009;4(3):535–541.
- Norata GD, Marchesi P, Pirillo A, . Long pentraxin 3, a key component of innate immunity, is modulated by high-density lipoproteins in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(5):925–931.
- Deban L, Jaillon S, Garlanda C, Bottazzi B, Mantovani A. Pentraxins in innate immunity: Lessons from PTX3. Cell Tissue Res. 2011;343(1):237–249.
- Arruda-Olson AM, Reeder GS, Bell MR, Weston SA, Roger VL. Neutrophilia predicts death and heart failure after myocardial infarction: A community-based study. Circ Cardiovasc Qual Outcomes. 2009;2(6):656–662.
- Rudiger A, Burckhardt OA, Harpes P, Muller SA, Follath F. The relative lymphocyte count on hospital admission is a risk factor for long-term mortality in patients with acute heart failure. Am J Emerg Med. 2006;24(4):451–454.
- Hurst RT, Ng DW, Kendall C, Khandheria B. Clinical use of carotid intima–media thickness: Review of the literature. J Am Soc Echocardiogr. 2007;20(7):907–914.
- Hornum M, Clausen P, Idorn T, Hansen JM, Mathiesen ER, Feldt-Rasmussen B. Kidney transplantation improves arterial function measured by pulse wave analysis and endothelium-independent dilatation in uraemic patients despite deterioration of glucose metabolism. Nephrol Dial Transplant. 2011;26(7): 2370–2377.
- Seyahi N, Cebi D, Altiparmak MR, . Progression of coronary artery calcification in renal transplant recipients. Nephrol Dial Transplant. 2012;27(5):2101–2107.
- Yilmaz MI, Qureshi AR, Carrero JJ, . Predictors of carotid artery intima–media thickness in chronic kidney disease and kidney transplant patients without overt cardiovascular disease. Am J Nephrol. 2010;31(3):214–221.
- Miyata T, van Ypersele de Strihou C, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: Origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int. 1999;55(2):389–399.
- Raj DS, Choudhury D, Welbourne TC, Levi M. Advanced glycation end products: A Nephrologist’s perspective. Am J Kidney Dis. 2000;35(3):365–380.
- Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011;63(1):102–110.
- Kodama S, Saito K, Yachi Y, . Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32(9):1737–1742.
- Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: A systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2010; 62(2):170–180.
- Kanbay M, Huddam B, Azak A, . A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8): 1887–1894.