Abstract
Objectives: This study evaluated the effects of a protocol aiming to reduce hypotension in acute kidney injury (AKI) patients submitted to sustained low-efficiency dialysis (SLED). Methods: Patients were randomly assigned to two SLED prescriptions—control group, dialysate temperature was 37.0°C with a fixed sodium concentration [138 mEq/L] and ultrafiltration (UF) rate; and profiling group, dialysate temperature was 35.5°C with a variable sodium concentration [150–138 mEq/L] and UF rate. Results: Sixty-two SLED sessions were evaluated (34 in profiling and 28 in control). Patients (n = 31) were similar in terms of gender, age, and Sequential Organ Failure Assessment (SOFA) score. Dialysis time, dialysis dose, and post-dialysis serum sodium were similar in both groups. The profiling group had significantly less hypotension episodes (23% vs. 57% in control, p = 0.009) and achieved higher UF volume (2.23 ± 1.25 L vs. 1.59 ± 1.03 L in control, p = 0.04) when compared with control group. Conclusions: SLED protocol with modulation of dialysate temperature, sodium, and UF profiling showed similar efficacy but less intradialytic hypotension when compared with a standard SLED prescription.
INTRODUCTION
The hemodynamic state is a critical factor for dialysis efficiency in acute kidney injury (AKI) patients. Currently, most of the AKI patients requiring renal replacement therapy (RRT) are critically ill and hemodynamically unstable. The RRT modalities preferentially used in this setting are continuous renal replacement therapies (CRRTs), such as hemofiltration and hemodiafiltration, or sustained low-efficiency dialysis (SLED).Citation1–3 Although SLED and CRRT have showed similar efficacy in AKI, SLED is less expensive and technically easier to perform.Citation1
Hypotension is a usual and feared event during RRT in critically ill AKI patients, jeopardizing its treatment. In addition, intradialytic hypotension may perpetuate ischemic injury and delay the renal function recovery in AKI.Citation4 Intradialytic hypotension is related to factors that are procedure dependent [volume and rate of ultrafiltration (UF), changes in plasma osmolality, composition, and temperature of dialysate] and patient dependent (hypovolemia, cardiac dysfunction, autonomic dysfunction, and vasodilation).Citation5
During SLED, the dialysate temperature is usually adjusted to 37°C, which increases the body temperature. This interferes with the hemodynamic response during UF and potentially induces and/or enhances hypotension. In patients with chronic kidney disease (CKD), the use of lower dialysate temperature reduced the number of episodes of hypotension during intermittent hemodialysis.Citation6–8 There are few data about the influence of dialysate temperature on hemodynamic parameters in patients with AKI, and most of the available information comes from CRRT studies.Citation9–12
Sudden modifications in plasma osmolality during RRT may cause changes in the interstitial fluid flow, favoring hypotension. The elevation of the dialysate sodium levels prevented intradialytic hypotension in CKD patients. However, it may promote hypernatremia, thirst, hypertension, and increased interdialytic weight gain.Citation13–17 This occurs especially when the sodium gradient (dialysate Na–patient Na) is positive.Citation16,17 However, recent data suggest lower mortality in CKD patients on dialysis with positive sodium gradient, possibly due to greater hemodynamic stability during hemodialysis.Citation18
Dialysate variable sodium concentrations (sodium profile) have been used in CKD patients preventing intradialytic hypotension, without the complications associated to high sodium concentrations in the dialysate. The efficacy of the sodium profile appears to be potentiated by the simultaneous use of an UF profile.Citation13 There is just one previous study that tested the simultaneous use of sodium and UF profile in RRT of AKI patients.Citation19
The objective of this study was to evaluate the efficacy of a protocol comprising reduced dialysate temperature associated to sodium and UF profile in the prevention of intradialytic hypotension in AKI critically ill patients submitted to SLED.
METHODS
Candidates participated in this study were patients older than 18 years admitted to the intensive care unit (ICU) with AKI requiring RRT. AKI was defined using the risk, injury, failure, loss, and end-stage kidney (RIFLE) classification criteria.Citation20 Patients with baseline serum creatinine >3 mg/dL, renal transplant recipients, and terminally ill patients (i.e., patients with therapeutically intractable diseases) were excluded.
The study protocol was approved by the Ethical Committee of the Sao Jose do Rio Preto Medical School and all patients, or their siblings, signed an informed consent before initiating the study.
Prevention of intradialytic hypotension episodes was the primary outcome evaluated.
SUSTAINED LOW-EFFICIENCY DIALYSIS
Block randomization was performed in groups of 20 patients. According to the computer-generated list, patients were randomly assigned to one of the RRT protocols described below.
Control
SLED, dialysate temperature of 37°C, fixed UF rate, and dialysate sodium concentration of 138 mEq/L.
Profile
SLED, dialysate temperature of 35.5°C, and sodium and UF profiles. The sodium profile had an initial sodium concentration of 150 mEq/L and a final concentration of 138 mEq/L, with a linear decrease in sodium concentration. The UF profiling pattern was chosen as it was programmed in the dialysis machine. In the pattern applied, the UF profile had a linear decrease in the UF rate, starting at 30% above the average UF rate.
SLED was performed using a Fresenius 4008B™ dialysis machine with volumetric UF control. The duration of SLED was 6–8 h. Blood flow was maintained between 150 mL/min and 200 mL/min and the dialysate flow was 300 mL/min. Filters with 1.8 m2 of area and an UF coefficient of 7.5 mL/h (Fresenius F8™) were used. The dialysate was generated with a proportioning system and water treated with a portable reverse osmosis machine. The standard composition of the dialysate was 35 mEq/L bicarbonate, 138 mEq/L sodium, 2.0 mEq/L potassium, and 3.5 mEq/L calcium.
Unfractionated heparin was used to prevent clotting of the extracorporeal circuit. Saline flushes (100–200 mL every 30 min) were used in patients with contraindication for anticoagulation with heparin. The nursing staff of the nephrology service was responsible for initiating and monitoring the dialysis procedure.
Hemodynamic Evaluation
Intradialytic hypotension was defined as a mean arterial pressure (MAP) below 60 mmHg, systolic arterial pressure below 90 mmHg, or the need for intervention (volume infusion, initiation, or increase of vasoactive drugs) to maintain blood pressure. The relative variation in blood volume was evaluated by a portable blood volume monitor (Crit-Line, Hema Metrics, Salt Lake City, UT, USA).
Severity Scores
The severity of the patients was evaluated according to the Sequential Organ Failure Assessment (SOFA)Citation21 score and Acute Tubular Necrosis Individual Severity Score (ATN-ISS)Citation22 on the day of the first dialysis.
SLED Dose
The SLED dose was assessed through the urea reduction ratio (URR) and single pool Kt/V (Daurgidas second-generation formula) based on the urea collected before and after dialysis. At the end of SLED, the dialysate flow was interrupted, blood flow was reduced to 50 mL/min, and blood was collected after 2 min to determine the urea level. The patients were weighed before and after SLED using a hospital bed scale (Soehnle Professional, Germany).
Statistical Analysis
The desired sample size was calculated for 40 patients per group (estimate of 4.5 hemodialysis sessions per patient) to give a power of 90%, and an alpha error of 0.05 to detect a 20% difference in the rate of hypotension between the treatment groups. The rate of hypotension in the control group was estimated to be 50% (in agreement with previous results from our institution) and 30% in the intervention group. As the estimated number of dialysis sessions per patient was uncertain, it was decided to analyze only patients who completed at least two dialysis sessions. Thus, there would be no bias in the statistical analysis for the group of patients who had made a greater number of dialysis sessions. The study was decided to discontinue early due to the results obtained in an interim data analysis.
Parametric variables were expressed as the average and standard deviation of the sample and were analyzed using an unpaired Student’s t-test. Nonparametric variables were represented as the median and 25–75% percentile and analyzed using the Mann–Whitney test. Categorical variables were expressed as the absolute (n) frequency and relative (%) frequency and were analyzed with the Pearson’s chi-square test or Fisher’s exact test, as appropriate. Differences were considered significant when p < 0.05. Statistical analysis was performed using GraphPad Prism 4 (GraphPad Software Inc., La Jolla, CA, USA).
RESULTS
Characteristics of the Studied Population
Except for the height, there were no significant differences in the characteristics of the patients in both groups, including disease severity evaluated by SOFA and ATN-ISS scores (). Sepsis was the most prevalent risk factor for developing AKI in both groups (65% in profile vs. 68% in control) followed by hypotension/hypovolemia and nephrotoxins (polymyxin, radiologic contrast, and myoglobin).
Table 1. Characteristics of the studied population.
Comparison of the Two Groups Regarding SLED-Related Parameters
A total of 147 SLED sessions were analyzed and conducted, 60 sessions with the 19 patients from the control group and 87 sessions with the 20 patients from the profile group. The final number of patients analyzed, with two or more SLED sessions, was 17 in the profile group (34 sessions) and 14 in the control group (28 sessions) (). At the time of first SLED, the profile group had a significantly higher potassium and glucose levels. The other laboratory parameters were similar between the two groups (). Patients in both groups had similar pre-SLED hemodynamic parameters, except for a slightly yet significantly higher MAP in the profile group (). This pattern was maintained during SLED (). When all patients were analyzed, regardless of the number of SLED sessions performed, patients in the control group still had lower MAP than the profile group (97 ± 22 mmHg in profile group vs. 86 ± 18 mmHg in control group, p < 0.01).
Figure 1. Study design and number of patients.
Notes: Control group: SLED, dialysate temperature 37.0°C, fixed Na and UF.Profile group: SLED, dialysate temperature 35.5°C, Na and UF profiling.
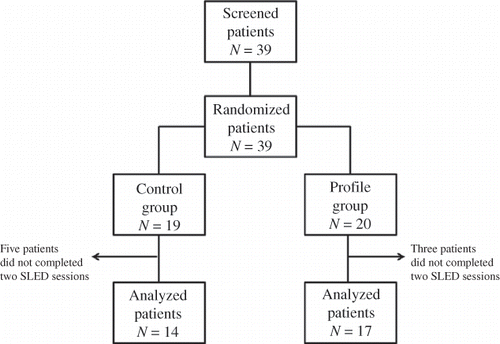
Figure 2. Mean arterial pressure during SLED.
Notes: Data are from the first and second SLED sessions.
Data are expressed as mean ± SEM.All points are different (p < 0.05), except from hour 5.
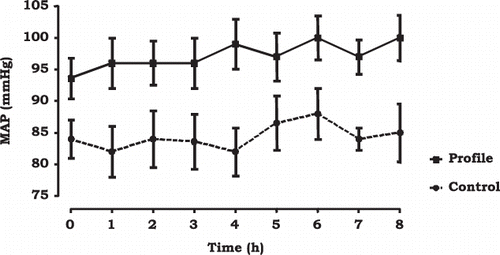
Table 2. Laboratory before the first SLED session.
Table 3. Hemodynamic data, SLED duration, ultrafiltration volume, and dose of the first and second SLED sessions.
Hypotension
Patients in the profile group had significantly fewer episodes of hypotension (). The duration of the SLED session and the ultrafiltrate volume are dependent on the SLED prescription and might contribute to a greater frequency of hypotension. However, the duration of SLED was similar in both groups, and patients in the profile group had actually a greater ultrafiltrate volume when compared with the control group (). Interestingly, the reduction in relative blood volume was similar in the two groups ().
Temperature, Sodium and SLED Dose
Patient’s temperature after SLED was lower in the profile group, but symptomatic episodes of hypothermia were not observed (). The sodium levels pre-SLED (control 144 ± 8 mEq/L vs. profile 143 ± 5 mEq/L; p = 0.13) and post-SLED (control 144 ± 7 mEq/L vs. profile 142 ± 4 mEq/L; p = 0.11) were similar in the two groups. The SLED dose was also similar in the two groups ().
In-Hospital Mortality and Hospital Length of Stay
The hospital mortality rate was similar for both groups: 80% in the profiling group and 89% in the control group (p = 0.66). The hospital length of stay was higher in the profile group [26 (15–40) days] when compared with the control group [15 (10–25) days], but this difference did not reach statistical significance (p = 0.14).
DISCUSSION
Hypotension is the principal complication of RRT in patients with AKI, particularly in those hospitalized in ICUs, and may occur in up to 70% of RRT sessions.Citation3,12
Although its obvious relevance, few studies have addressed the prevention strategies against intradialytic hypotension in AKI patients. The approaches that have been suggested are adapted from procedures used in CKD population.
In a review article, Murray and Doshi suggested optimization of fluid removal, improvement of vascular contractility, and improvement of cardiac function as possible strategies for the prevention of hypotension during dialysis in AKI.Citation5 These authors considered that the prescription of intermittent hemodialysis in intensive care patients should routinely include a reduction of dialysate temperature, sodium profile, and elevated calcium concentrations in the dialysate. They recommend using SLED or CRRT in patients with hemodynamic instability or when the extracellular volume cannot be reduced by intermittent hemodialysis. We incorporated several of these suggestions in this study, more specifically optimization of fluid removal (UF profile), dialysate temperature reduction, and sodium profile in the experimental group. High dialysate calcium concentration was used either in control or in the experimental groups.
The use of a protocol-based SLED in this study was associated with significantly less intradialytic hypotensive episodes in unstable critically ill AKI patients. This is a highly relevant clinical finding, since it showed that a protocol-based SLED is feasible and effective in severely ill AKI patients, who most of the time would be treated by CRRT methods. This was not a primary outcome for this study, but it should be noted that the protocol-based SLED did not influence AKI mortality. In fact, even studies specifically designed to address this issue achieved mostly neutral results when assessing the effects of type, timing, and dosing of RRT on AKI mortality.Citation1–3
This study has some limitations. It is a single-center study. The achieved SLED dose by session was approximately 20% smaller than the targeted one, a phenomenon frequently seen in critically ill AKI patients on RRT.Citation23 Finally, although both groups had similar systolic blood pressure (SBP), the control group had lower MAP before and during SLED. However, both groups had blood pressure above the recommended levels for critically ill patients (>65 mmHg) and similar frequency of vasoactive drugs use, indicating similar hemodynamic status, which makes unlike that this slight difference in the pre-SLED MAP had influenced the frequency of hypotensive episodes during SLED. Moreover, the study aim was to assess the frequency of episodes of hypotension during SLED, not absolute blood pressure values.
To the best of our knowledge, there are only two studies assessing the effects of protocols for preventing intradialytic hypotension in AKI patients. Schortgen et al. implemented several procedures to prevent hypotension in patients undergoing intermittent hemodialysis in ICUs.Citation12 They suggested prescribing RRT with modified cellulose membranes, simultaneous connection of the extracorporeal circuit lines, dialysate sodium concentration ≥145 mEq/L, dialysate temperature ≤37°C, blood flow of 150 mL/min, and a minimum duration of 4 h per RRT session. In patients with hemodynamic instability, they recommended that the dialysate temperature should be 35°C. The authors demonstrated a reduction of intradialytic hypotension episodes from 71% to 61%. Adherence to the protocol varied according to the suggested procedure. Dialysate with reduced temperature and Na+ ≥ 145 mEq/L were used in only 13% and 67% of the dialysis sessions, respectively. In contrast, in this study all patients in the intervention group received the full set of measures prescribed, which resulted in fewer episodes of hypotension.
Paganini et al. evaluated the effect of sodium profile (160–140 mEq/L) and UF (50% during the first third and 50% in the last two thirds of the RRT) on the hemodynamic status of 10 AKI patients undergoing intermittent hemodialysis.Citation19 After three RRT sessions with Na+ profile and UF, the patient was switched to a conventional dialysis, that is, dialysate with Na+ 140 mEq/L and a fixed UF rate. The UF volume was greater in sessions with a Na+/UF profile, and the frequency of hypotension was 16% in the profile group compared with 45% in the control group.
In conclusion, the use of a protocol-based SLED (lower dialysate temperature associated to sodium and UF profiles) in critically ill AKI patients reduced significantly the episodes of intradialytic hypotension, achieved significantly higher UF rate, and provided similar RRT dose when compared with a standard SLED prescription. These results indicate that SLED with a reduced dialysate temperature and sodium and UF profiles is a safe, efficient, and feasible way to perform RRT in critically ill AKI patients.
ACKNOWLEDGMENTS
The authors are very grateful to the patients who granted permission to inclusion in the study and to the nephrology nursing staff for carrying out the RRT procedures.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
This project was supported by a grant (04/10,727-6) from FAPESP, Brazil.
EAB is partially supported by a grant from CNPq, Brazil.
EQL was partially supported by a grant from the Sao Jose Rio Preto Medical School.
REFERENCES
- , VA/NIH Acute Renal Failure Trial NetworkPalevsky PM, Zhang JH, O’Connor TZ, . Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20.
- , RENAL Replacement Therapy Study InvestigatorsBellomo R, Cass A, Cole L, . Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638.
- Vinsonneau C, Camus C, Combes A, . Continuous venovenous hemodiafiltration versus intermittent hemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicenter randomized trial. Lancet. 2006;368:379–385.
- Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–310.
- Murray PT, Doshi M. Approach to intradialytic hypotension in intensive care unit patients with acute renal failure. Artif Organs. 2003;27:772–780.
- Maggiore Q, Pizzarelli F, Santoro A, . Study Group of Thermal Balance and Vascular Stability. The effects of control of thermal balance on vascular stability in hemodialysis patients: results of the European randomized clinical trial. Am J Kidney Dis. 2002;40:280–290.
- van der Sande FM, Kooman JP, Konings CJ, Leunissem KM. Thermal effects and blood pressure response during postdilution hemodiafiltration and hemodialysis: the effect of amount of replacement fluid and dialysate temperature. J Am Soc Nephrol. 2001;12:1916–1920.
- Cruz DN, Mahnensmith RL, Brickel HM, Perazella MA. Midodrine and cool dialysate are effective therapies for symptomatic intradialytic hypotension. Am J Kidney Dis. 1999;33:920–926.
- Yagi N, Leblanc M, Sakai K, Wright EJ, Paganini EP. Cooling effect of continuous renal replacement therapy in critically ill patients. Am J Kidney Dis. 1998;32:1023–1030.
- Matamis D, Tsagourias M, Koletsos K, . Influence of continuous hemofiltration-related hypothermia on hemodynamic variables and gas exchange in septic patients. Intensive Care Med. 1994;20:431–436.
- Rokyta RJr, Matejovic M, Krouzecky A, Opatrny KJr, Ruzicka J, Novak I. Effects of continuous venovenous hemofiltration-induced cooling on global hemodynamics, splanchnic oxygen and energy balance in critically ill patients. Nephrol Dial Transplant. 2004;19:623–630.
- Schortgen F, Soubrier N, Delclaux C, . Hemodynamic tolerance of intermittent hemodialysis in critically ill patients. Am J Respir Crit Care Med. 2000;162:197–202.
- Song JH, Park GH, Lee SY, Lee SW, Lee SW, Kim MJ. Effect of sodium balance and the combination of ultrafiltration profile during sodium profiling hemodialysis on the maintenance of the quality of dialysis and sodium and fluid balances. J Am Soc Nephrol. 2005;16:237–246.
- Penne EL, Levin NW, Kotanko P. Improving volume status by comprehensive dietary and dialytic sodium management in chronic hemodialysis patients. Blood Purif. 2010;30:71–78.
- Meira FS, Figueiredo AE, Zemiarcki J, Pacheco J, Poli de Figueiredo CE, d’Avila DO. Two variable sodium profiles and adverse effects during hemodialysis: a randomized crossover study. Ther Apher Dial. 2010;14:328–333.
- Munoz Mendoza J, Sun S, Chertow GM, Moran J, Doss S, Schiller B. Dialysate sodium and sodium gradient in maintenance hemodialysis: a neglected sodium restriction approach? Nephrol Dial Transplant. 2011;26:1281–1287.
- Santos SF, Peixoto AJ. Sodium balance in maintenance hemodialysis. Semin Dial. 2010;23:549–555.
- Hecking M, Karaboyas A, Saran R, . Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2012;59:238–248.
- Paganini EP, Sandy D, Moreno L, Kozlowski L, Sakai K. The effect of sodium and ultrafiltration modeling on plasma volume changes and hemodynamic stability in intensive care patients receiving hemodialysis for acute renal failure: a prospective, stratified, randomized, cross-over study. Nephrol Dial Transplant. 1996;11(Suppl. 8):32–37.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative Workgroup. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8(4):R204–R212.
- Vincent JL, Moreno R, Takala J, . Sepsis-Related Organ Failure Assessment score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710.
- Liaño F, Gallego A, Pascual J, . Prognosis of acute tubular necrosis: An extended prospectively contrasted study. Nephron. 1993;63:21–31.
- Evanson JA, Himmelfarb J, Wingard R, . Prescribed versus delivered dialysis in acute renal failure patients. Am J Kidney Dis. 1998;32:731–738.
