Abstract
Renal failure is common in patients with glomerular disease. Although renal failure may result from the glomerular lesion itself, it is also observed in patients with minimal glomerular alterations. Degenerative changes and necrosis of the tubular epithelium are common findings in kidney biopsies from these patients. The aim of this work is to examine the association between acute tubular necrosis (ATN) and renal failure in patients with glomerulopathy and to estimate the relationship between the degree of ATN and renal failure in these patients. Data on age, sex, presence of nephrotic syndrome, and renal failure were recorded for 149 patients, who underwent a renal biopsy for the diagnosis of glomerulopathy. The biopsies were reviewed, and ATN, when present, was classified as one of four grades depending on its intensity. The mean age of the patients was 21 ± 16 years. Eighty patients (54%) were male, 43 (42%) had renal failure, 104 (72%) had nephrotic syndrome, and 66 (45%) had minimal change disease or focal segmental glomerulosclerosis. ATN was present in 115 (77%) patients. The frequency of renal failure was directly correlated with the intensity of ATN [odds ratio (OR) of 26.0 for patients with grade 2 lesions and OR of 45.5 for patients with grade 3 lesions]. ATN is a common finding in the biopsies of patients with glomerulopathy. The severity of ATN is directly associated with the frequency of renal failure in these patients.
INTRODUCTION
Acute kidney injury (AKI) is a common complication in patients with glomerular disease and is a hallmark of diffuse proliferative or crescentic glomerulonephritis.Citation1 However, the association between AKI and glomerular disease has also been reported in patients with minimal change disease.Citation2 Although infections, nephrotoxic drugs, and renal vein thromboses are precipitating conditions for AKI in these patients,Citation3 no direct cause of AKI can be identified in a significant number of patients with glomerular disease.Citation4
For instance, no studies have identified any histological changes that could cause an acute decrease in the glomerular filtration rate in the kidneys of most of the patients with AKI and idiopathic nephrotic syndrome. On the other hand, acute tubular necrosis (ATN) has been observed in adults with minimal change disease and AKI.Citation5,6 In some cases, the association of nephrotic syndrome and ATN may suggest a rapidly progressive glomerulonephritis that leads to the early institution of immunosuppressive therapy.Citation7
The aims of this study are to examine the association between ATN and renal failure in patients with glomerulopathy, to estimate the frequency of ATN in patients with glomerulopathies, and to estimate the correlation between the histological intensity of ATN and the frequency of renal failure in these patients.
MATERIALS AND METHODS
Renal Biopsies
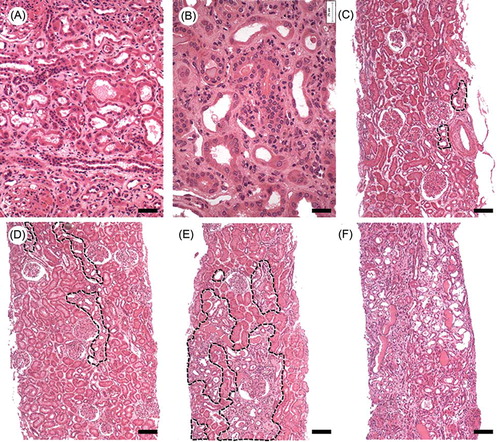
Renal biopsies performed for the diagnosis of glomerular diseases were examined at the Gonçalo Moniz Research Center—Fiocruz, Salvador, BA, Brazil, between January 2003 and December 2009. The biopsies were classified according to the intensity of the ATN: grade 0, absent; grade 1, rare necrotic tubules dispersed throughout the cortex; grade 2, small groups of necrotic tubules discontinuously distributed throughout the renal cortex; grade 3, coalescent groups of necrotic tubules easily found in the renal cortex; and grade 4, extensive areas of tubular necrosis scattered throughout the renal cortex (). The following tubular changes were considered to be evidence of either current or recent ATN: tubular dilatation; thinning of the tubular epithelium; cellular casts; interstitial edema; and the presence of morphological markers of epithelial regeneration such as cellular hyperchromatism, mitosis, and binucleation. In all instances, the renal specimens were obtained by percutaneous biopsies, fixed in Bouin’s solution, paraffin embedded, cut at 2-μm thickness, and stained with hematoxylin and eosin. Cases were excluded if their respective slides were not available for histological review, contained insufficient renal cortical tissue, or presented artifacts that could interfere with the estimation of ATN. The glomerulopathy diagnosis was defined as that recorded in the pathology report.
Figure 1. Representative histology of the tubular changes considered to be evidence of ATN (A) and (B) and of the four grades attributed to the lesion (C)–(F): (A) tubular dilatation, thinning of the tubular epithelium, interstitial edema; (B) cellular hyperchromatism, mitosis, and binucleation; (C) ATN grade 1: only rare tubules with evidence of necrosis are observed in the cortex; (D) ATN grade 2: small groups of necrotic tubules discontinuously distributed throughout the renal cortex; (E) ATN grade 3: groups of necrotic tubules are easily found in the renal cortex; (F) ATN grade 4: extensive areas of tubular necrosis are scattered throughout the renal cortex. ATN grade 0 (normal) not represented. Bar = (A), 50 μm; (B), 25 μm; and (C)–(F), 100 μm.
Clinical Data
The following data were obtained from referral forms submitted with the renal specimens: age, gender, diagnosis of nephrotic syndrome, serum creatinine levels, and diagnosis of renal failure. For the purpose of this study, patients were diagnosed with nephrotic syndrome when the reported urinary protein excretion was greater than 3.5 g/24 h or when this diagnosis was reported by the nephrologist who requested the biopsy. In the routine of the hospitals involved in the study, the diagnosis of renal failure was usually based on the increase in creatinine levels or on the decrease in urinary output. Hence, we considered evidence of renal failure in the patients of the study, the presence of serum creatinine levels greater than 1.2 mg/dL for children and women or above 1.5 mg/dL for men, or when this diagnosis was reported in the request form. Children were defined as patients who were younger than 16 years of age.
Accuracy and Reproducibility of Histological Classification
To estimate the accuracy of the semi-quantitative grading of ATN, randomly selected cases underwent morphometric analysis using two methodologies: (1) estimating the number of necrotic tubules per cortical area (35 cases) or (2) measuring the percentage of the cortical area affected by ATN (19 cases). Both procedures were performed with semi-automated morphometry using a camera that was attached to the light microscope (CX41, Olympus, Tokyo, Japan) and Image-Pro Plus software version 6.0 (MediaCybernetics, Bethesda, MD, USA).
To test the intraobserver reproducibility of the semi-quantitative analyses, 50 randomly selected cases of the study were reviewed by the pathologist 3 months after the initial examination.
Statistical Analysis
Data are expressed as percentages and absolute numbers and are summarized as the means ± standard deviations or as medians and the 25th and 75th percentiles. Comparisons between groups were performed with the chi-square test. The Spearman correlation test was used to assess the correlation between the morphometric and semi-quantitative analyses of ATN and between the intensity of ATN and renal failure. The concordance between categorical results was evaluated using the kappa coefficient calculated according to Landis and Koch.Citation8 The associations between renal failure and ATN were tested by calculating the odds ratios (ORs) with 95% confidence intervals. The results were considered to be statistically significant if p < 0.05. Data were analyzed using Prism 5.01 (GraphPad, San Diego, CA, USA) and StataIC11 software (StataCorp LP, College Station, TX, USA).
Ethical Considerations
The study was conducted in accordance with resolution No. 196/96 of the National Health Council, and the procedures were approved by the Ethics Committee for Research Involving Human Subjects of Centro de Pesquisa Gonçalo Moniz, Fiocruz, Protocol No. 188/09.
RESULTS
A total of 149 cases, 80 males and 67 females (the information was not available for two patients), were reviewed (). The mean age of the patients was 21 ± 16 years, and patients’ ages ranged from 1 to 75 years. Fifty-two patients (36%) were children. Nephrotic syndrome was diagnosed in 104 (72%) patients and the most frequent histological diagnosis was focal and segmental glomerulosclerosis (FSGS) followed by minor glomerular abnormalities (mostly associated with nephrotic syndrome) ().
Table 1. General characteristics of the patients undergoing renal biopsy for suspected glomerulonephritis in nephrology referral hospitals in Salvador, BA, in the period 2003–2009.
Renal failure was present in 42% of the patients for whom renal function data were reported and ATN was observed in 115 (77%) patients ().
There was no significant difference between adults and children with respect to gender, most frequent clinical syndrome (in both groups was nephrotic syndrome), the frequency of renal failure, or the percentage of ATN (). Although the frequency of ATN was high in both groups, as shown in most of the patients had tubular necrosis grade 1 (rare isolated foci) or grade 2 (small noncontiguous areas).
Figure 2. The distribution of the different degrees of ATN in adults and children with glomerular diseases.
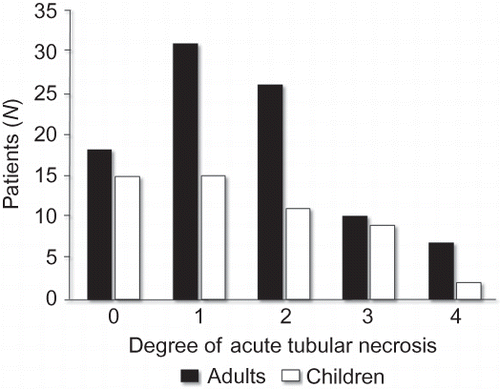
Table 2. Comparison between adults and children undergoing renal biopsy for suspected glomerulonephritis in nephrology referral hospitals in Salvador, BA, in the period 2003–2009.
The attributed severity of ATN, in four degrees, had a strong association with both the morphometric estimate of the number of necrotic tubules per cortical area (r = 0.88, p < 0.0001) and the morphometric estimate of the percentage of cortical area affected by necrotic tubules (r = 0.93, p < 0.0001). This finding indicated that the subjective estimate was robust enough to be used in the analysis proposed in this study.
The frequency of different grades of ATN did not differ significantly between children and adults ().
Renal failure was observed in 51 (53%) out of 97 patients with ATN and in 2 (7%) out of 28 patients without ATN (p < 0.0001). The frequency of patients with renal failure increased with increasing ATN grade (): renal failure was observed in 2 of 28 (7%) patients without ATN; in 10 of 38 (26%) patients with grade 1 ATN; in 22 of 33 (67%) patients with grade 2 ATN; in 14 of 18 (78%) patients with grade 3 ATN; and in 5 of 8 (62%) patients with grade 4 ATN. The frequency of renal failure was significantly higher for patients with grade 2 (26.0, p < 0.0001) or grade 3 (45.5, p < 0.0001) ATN than for patients without ATN ().
Table 3. Association between the degree of ATN and ARF in patients with glomerulopathy.
The intraobserver reproducibility of the semi-quantitative estimate of ATN, which was performed by the revaluation of 50 cases 3 months after the first analysis, showed a moderate degree of agreement (κ = 0.53, p < 0.001). Furthermore, the kappa coefficient was 0.76 (p < 0.001) when the number of ATN grades was reduced by aggregating the grades based on clinical relevance, i.e., grouping grades 0 and 1 and grades 2–4.
DISCUSSION
In this study, we found that clinical and laboratory signs of renal failure were reported in almost half of patients with glomerular diseases. Although in some of these patients inflammatory glomerular lesions may contribute to the renal failure, inflammatory lesions with diverse severity were present in approximately 15% of our casuistic. Conversely, histological findings consistent with ATN were present in 77% of the patients and the severity of ATN correlated with the finding of renal failure. Variable severity of inflammatory glomerular lesions that may contribute to the renal failure, however, was found in approximately 15% of the biopsies.
The definition of renal failure used in this study was based on the elevation of serum levels of creatinine above the conventional normal levels. It has been shown that creatinine is a late marker of decrease in the glomerular filtration rateCitation9 and is not specific for AKI. However, recent studies to standardize the diagnosis of AKI use the increase in serum creatinine levels over baseline value as a definition of this condition.Citation10,11 Furthermore, subsequent studies validated abrupt increase in serum creatinine as a criterion for diagnosis of AKI.Citation12,13 Since our investigation represents a cross-sectional study, serial creatinine levels and other features that could differentiate prerenal azotemia and ATN like fractional excretion of sodium, urinary sediment, and osmolality were not always evaluated or provided in the renal biopsy referral forms; similarly, data on outcome of renal function were not available, representing a limitation of the study. Nevertheless, we found a strong association between the presence of ATN on histologic examination and the clinical diagnosis of renal failure in these patients, suggesting the acute character of the renal dysfunction.
Recently, much emphasis has been placed on the dissociation between clinical renal dysfunction and histological findings.Citation14 Nevertheless, histological evidence of ATN is widely used in the follow-up kidney transplantation.Citation15 This study supports this practice in the study of biopsies of native kidneys and demonstrates that the finding of tubular necrosis, even when it occurs in a patchy and irregular distribution that may be easily overlooked or missed by typical needle biopsies, is associated with an increased risk of AKI in patients with renal disease. This finding suggests that these discrete lesions may also be implicated in some cases of so-called idiopathic AKI observed in patients with nephrotic syndrome. In fact, using a semi-quantitative estimate of ATN, we observed that the evidence of tubular necrosis was common even in those patients with minimal glomerular alterations. The prevalence of patients with renal failure was directly correlated with the severity of ATN. Furthermore, in the present analysis, we found that even mild tubular lesions (grade 2) were associated with significant increases in the frequency of renal failure. In some experimental studies, the high susceptibility of S3 tubular segment to ischemic and nephrotoxic lesion has been highlighted.Citation16,17 Unfortunately, given the variable representation of the outer medullary stripe in human biopsies, a systematic analysis of this area was not included in this study. The evaluation of this region in human biopsies using an appropriate design may refine the conclusions achieved by this study.
The most frequent glomerulopathy diagnoses in the patients were FSGS and minimal change disease, which is similar to previous studies.Citation18,19 Nephrotic syndrome, a common finding in patients with glomerulopathies, was diagnosed in 72% of the patients.
Reversible renal failure associated with nephrotic syndrome has been reported mainly in adults with minimal change disease.Citation20 Waldman et al.Citation21 reported acute renal failure (ARF) in 25.3% of the 95 adult patients with minimal change disease reviewed: more frequently they were male and tended to be older, hypertensive, and with lower serum albumin and more severe proteinuria than those without ARF. Mean time to recover renal function was 6 weeks. At follow-up, however, the patient with previous ARF had higher serum creatinine than patients without ARF. Interestingly, ATN, which histologically resembles ischemic injury, has been reported in some patients with ARF and minimal change disease.Citation5 The pathophysiology of the acute tubular injury observed in glomerulopathies is not well understood. Several conditions could be associated with the development of AKI in patients with nephrotic syndrome, including the use of nephrotoxic drugs, renal vein thromboses, intravascular volume depletion, hypotension, and interstitial nephritis. However, in a considerable number of patients, no etiologic factor has been identified, and the AKI is considered idiopathic.Citation3,22
Although Jennette and FalkCitation5 correlated renal failure in patients with nephrotic syndrome with old age, the presence of arteriolosclerosis, and conditions that can promote ischemic renal injury, in this study, we observed no difference in the frequency of renal failure between adults and children. One possible explanation for the similar rates of ATN-associated renal failure in children and adults could be related to the mean age of the patients in our series (24 ± 16 years), with 75% of the patients under 30 years of age, which is lower than the mean ages reported in the literature (approximately 40 ± 16). Such an observation strongly suggests that ATN associated with nephrotic syndrome is not necessarily associated with vascular degenerative conditions. Additional studies in patients with minimal change disease are necessary to better evaluate the influence of age, severity of proteinuria and serum levels of albumin, associated hypertension, and the treatment of the nephrotic syndrome in the pathogenesis of ATN and recovery of renal function in these patients. A study aiming this particular group of patients is now under way in our laboratory.
Finally, the graded classification of ATN used in our study was strongly correlated with morphometric analyses of either the number of necrotic tubules per cortical area or the percentage of the cortical area containing necrotic tubules. In addition to demonstrating substantial intraobserver agreement in the severity of ATN and its correlation with the clinical finding of renal failure, these data support the use of this classification in routine histological diagnosis, without the need for additional equipment and technical resources. The early identification of ATN in patients with glomerulopathies may prevent the use of inappropriate immunosuppressive drugs, thereby avoiding their potentially dangerous consequences.Citation7
ACKNOWLEDGMENT
We thank the staff of the nephrology referral services of Salvador, BA, particularly Dr. Marilia B. de Oliveira from the Hospital Ana Nery; Dr. Marcia F. Carneiro and Dr. Rilma Santos from the Hospital Roberto Santos General; and Dr. Marcia Conrado from the Hospital Santo Antonio.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Prakash J, Sen D, Kumar NS, Kumar H, Tripathi LK, Saxena RK. Acute renal failure due to intrinsic renal diseases: Review of 1122 cases. Ren Fail. 2003;25(2):225–233.
- Chamberlain MJ, Pringle A, Wrong OM. Oliguric renal failure in the nephrotic syndrome. Q J Med. 1966;35(138):215–235.
- Cavagnaro F, Lagomarsino E. Peritonitis as a risk factor of acute renal failure in nephrotic children. Pediatr Nephrol. 2000;15(3–4):248–251.
- Koomans HA. Pathophysiology of acute renal failure in idiopathic nephrotic syndrome. Nephrol Dial Transplant. 2001; 16(2):221–224.
- Jennette JC, Falk RJ. Adult minimal change glomerulopathy with acute renal failure. Am J Kidney Dis. 1990;16(5):432–437.
- Lowenstein J, Schacht RG, Baldwin DS. Renal failure in minimal change nephrotic syndrome. Am J Med. 1981;70(2): 227–233.
- Rodamilans MF, Barros LL, Carneiro MM, dos Santos WL, Rocha PN. Challenges in clinical-pathologic correlations: Acute tubular necrosis in a patient with collapsing focal and segmental glomerulosclerosis mimicking rapidly progressive glomerulonephritis. Ren Fail. 2010;32(8):1005–1008.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174.
- Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24(11):3263–3265.
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334(22):1448–1460.
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8(4):R204–R212.
- Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23(4): 1203–1210.
- Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008; 73(5):538–546.
- Rosen S, Stillman IE. Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol. 2008;19(5):871–875.
- Solez K, Morel-Maroger L, Sraer JD. The morphology of “acute tubular necrosis” in man: Analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore). 1979;58(5):362–376.
- Gueler F, Rong S, Park JK, . Postischemic acute renal failure is reduced by short-term statin treatment in a rat model. J Am Soc Nephrol. 2002;13(9):2288–2298.
- Nosaka K, Nakada J, Endou H. Cisplatin-induced alterations in renal structure, ammoniagenesis and gluconeogenesis of rats. Kidney Int. 1992;41(1):73–79.
- dos-Santos WL, Sweet GM, Bahiense-Oliveira M, Rocha PN. Schistosomal glomerulopathy and changes in the distribution of histological patterns of glomerular diseases in Bahia, Brazil. Mem Inst Oswaldo Cruz. 2011;106(7): 901–904.
- Polito MG, de Moura LA, Kirsztajn GM. An overview on frequency of renal biopsy diagnosis in Brazil: Clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol Dial Transplant. 2010;25(2):490–496.
- Smith JD, Hayslett JP. Reversible renal failure in the nephrotic syndrome. Am J Kidney Dis. 1992;19(3):201–213.
- Waldman M, Crew RJ, Valeri A, . Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2(3):445–453.
- Furuya R, Kumagai H, Ikegaya N, . Reversible acute renal failure in idiopathic nephrotic syndrome. Intern Med. 1993;32(1):31–35.