Abstract
Cadmium is a widespread, toxic industrial pollutant. The proximal tubule of the mammalian kidney is a major target of Cd-induced toxicity. We analyzed the effects of cadmium exposure on the model system of experimental animals, the thiobarbituric acid (TBA)-reactive substance (TBARS) level, and the activity of xanthine oxidase (XO) and catalase in kidney of rats, with and without glutathione and lipoic acid (LA). The experimental animals were classified into six groups, regarding cadmium, glutathione, and LA intake. The concentration of TBARSs in the homogenate was determined by spectrophotometric method according to Nabavi et al. The specific activity of XO was determined spectrophotometrically by the method of Aygul et al. Catalase activity in tissues was determined by spectrophotometric method according to Nabavi et al. The increased level of TBARS and the increased activity of XO in kidney tissue in cadmium poisoning are statistically significant compared to control (p < 0.001). Glutathione and LA applied along with cadmium lowered TBARS concentration and reduced XO activity (p < 0.001). Catalase activity in the kidney tissue was increased in the group, which was administered cadmium (p < 0.001). In conclusion, glutathione and LA, as physiological antioxidants applied with cadmium, have reduced the level of lipid peroxide and the activity of XO, and can be used as protectors in conditions of cadmium poisoning.
INTRODUCTION
Cadmium is a widespread highly toxic industrial pollutant. According to the US Environmental Protection Agency, it is listed as one of the 126 most dangerous environmental pollutants. Each year, around 13,000 tons of this heavy metal are being produced and released in the environment.Citation1 The major sources of cadmium intake are contaminated drinking water, food, and tobacco smoke.
A natural cause of occasional increase in cadmium concentration in the air is volcanic activity. Artificial increase in cadmium intake is associated with black metallurgy and usage of fossil fuels. Due to the fact that artificial phosphate fertilizers contain cadmium, the intake of cadmium through food is thought to be the most significant source of cadmium poisoning in nonsmokers.Citation2
Products containing cadmium are rarely recycled, often inappropriately discarded, and burned so that the risk for human health had been dramatically increasing during the twentieth century.Citation3
Smoking is one of the most important sources of cadmium intake. It has been proved that cadmium concentration in a smoker’s blood is 4–5 times higher when compared to nonsmokers.Citation4 High toxicity of this heavy metal is associated with the fact that its half-life is between 15 and 20 years.Citation5
The proximal tubule cells of the mammalian kidney are the major targets of Cd poisoning.Citation6 Cd-induced nephrotoxicity is thought to be mediated through the Cd–metallothionein (CdMT) complex, which is synthesized in the liver, released into the circulation, and taken up by renal tubule cells.Citation7
Although cadmium is not a redox-active metal and therefore is not able to participate directly in Fenton’s reaction and the creation of reactive oxygen species (ROS), it is able to induce oxidative stress and to cause consequent damage to body structures through indirect mechanisms.Citation8
Lipid peroxidation is the most significant negative consequence of ROS generation, which ends up irreversibly damaging the function and structure of cell membrane. Thiobarbituric acid (TBA)-reactive substances (TBARSs) are the final products and indicators of lipid peroxidation level.Citation9
One of the bases of cadmium toxicity is its effect on enzymatic cell systems due to the exchange of their metal ions (mostly Zn2+ and Cu2+) in metalloenzymes and a very high affinity to biological structures containing SH groups.Citation10,11 Some of the important enzymes whose activity is affected by cadmium are those that participate in the metabolism of xenobiotics.Citation12 The oxidation reactions are probably the common stage I in the xenobiotic transformation, and xanthine oxidase (XO) is an enzyme included in this process.
XO is an enzyme present in two interconvertible forms: dehydrogenase and oxidase. The majority of researches agree that XO dehydrogenase form is converted to oxidase form through the mechanism which includes oxidation of sulfhydryl groups or limited proteolysis of enzyme molecule itself, thereby creating an enzyme able to produce hydrogen peroxide and superoxide.Citation12,13
Catalase (EC 1.11.1.6, hydrogen peroxide oxidoreductase) is a heme-containing tetramer, which catalyzes decomposition of hydrogen peroxide into water and molecular oxygen. It is, therefore, one of the primary antioxidative enzymes that take part in the protection from oxidative stress caused by cadmium poisoning.Citation14
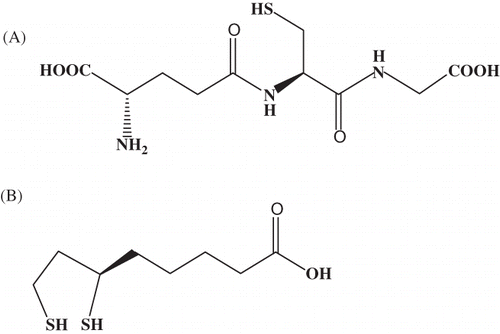
Glutathione (GSH, l-γ-glutamyl-l-cysteinylglycine) is a tripeptide (A), which makes up to 90% of all nonprotein sulfidic cell compounds and is an essential cofactor of some enzymes.Citation15
Due to the fact that GSH molecule contains thiol group through which it forms a mercaptide bond with heavy metal ions such as Zn, Cu, Cd, Pb, and Ag, it acts as a chelating agent. The most probable chemical structure (formula) of GSH—heavy metal ion complex—is M(GSH)2. The stability of this complex depends on metal ion size, acid–base characteristics, and affinity of metal ions to thiol group that decreases in line: Cd2+ > Pb2+ > Cu+/Cu2+.Citation16
Lipoic acid (LA) is a cyclic disulfide (B) linked via carboxylic group with the protein part of an enzyme molecule forming amide bound. It is a powerful natural antioxidant whose antioxidant effects are the object of clinical studies in the treatment of diabetic micro- and macroangiopathy.Citation17,18 New data point to potential preventive effects in the development of Alzheimer’s disease, atherosclerosis, hypertension, coronary disease, and heavy metal poisoning.
LA participates in oxidative decarboxylation of 2-oxyacids, while reduced –SH groups represent locations able to bond heavy metal ions easily. The effect of LA in the condition of cadmium poisoning has not yet been tested.
The mechanisms underlying Cd-induced nephrotoxicity remain poorly understood, both at the cellular level and in vivo.Citation19 Although much is known about oxidative stress caused by cadmium, there are no experimental studies of the effect of cadmium on XO oxidative activity. Catalase activity in 7-day cadmium poisoning is also not known.
The aim of this study was to test the effect of cadmium on TBARS concentration as well as the activity of XO and catalase in the kidney of rats and to determine the possible protective effect of GSH and LA in the given condition.
EXPERIMENTAL PART
Chemicals
The reagents were purchased from Sigma (St. Louis, MO, USA) and were of the highest commercial quality available. All used chemicals were of analytical grade.
Animals
A model system, for testing the effects of exposure to cadmium in industrial workers and in general, was the study conducted in 6-week-old female albino Wistar rats, weighing from 150 to 200 g.Citation20
Rats were purchased from the farm of Military Medical Academy (Belgrade, Serbia) and bred in the vivarium of the Faculty of Medicine, University of Nis, under standard conditions. The animals were housed inside a well-ventilated room, kept at temperature of 23 ± 2°C with 55 ± 10% humidity and 12 h light:dark cycle (07.00–19.00 h). The animals were given a commercial rat diet and tap water ad libitum. Animals used for the procedure were treated in accordance with the NIH Guide for Care and Use of Laboratory Animals (1985).
All experiments on animals were performed according to guidelines and had been approved by the Animal Ethics Board at the University of Nis Medical School beforehand.
Treatment Procedure
The animals were divided into six groups:
| • | Control group in which the rats were administered with (physiological) saline solution (0.9%) intraperitoneally for 21 days with break on every third day. | ||||
| • | Cadmium group in which the rats subcutaneously (sc.) received sublethal dose of cadmium with total concentration of 0.54 mg CdCl2 per animal, on every third day for 7 days. | ||||
| • | Cadmium and LA group in which the rats were administered (sc.) with sublethal dose of cadmium on every third day for 7 days, as well as LA on every second day during the experiment. | ||||
| • | Cadmium and GSH group in which the rats were administered (sc.) with sublethal dose of cadmium on every third day for 7 days, as well as GSH on every second day during the experiment. | ||||
| • | GSH group in which the rats were administered with 2.4 mg of GSH (per rat) for 21 days with break on every third day. | ||||
| • | Lipoic acid group in which the rats were administered with 2.4 mg of LA (per rat) for 21 days with break on every third day. | ||||
Anesthesia and Tissue Collection
At the end of the treatment period, animals were anesthetized with ketamine (60 mg/kg) given intraperitoneally and then sacrificed. Their kidneys were removed and frozen to −20°C.
Homogenate Preparation
The kidney tissue was cut in small pieces and homogenized in ice cold water, using homogenizer (IKA® Works do Brasil Ltda, Rio de Janeiro, Brasil). The homogenates (10% w/v) were centrifuged at 1500×g for 10 min at 4°C. The resulting supernatant was separated and used for the biochemical analysis.
Biochemical Analysis
Lipid peroxidation products
Lipid peroxidation in terms of TBARS formation was determined using slightly modified method of Nabavi et al.Citation21 The homogenate was treated with trichloroacetic acid and TBA and was kept in a boiling water bath for 30 min. The amount of TBARSs formed was measured at 532 nm against blank. Concentration was expressed in nmol malondialdehyde eq/g tissue of protein.
XO activity
Specific activity of XO was evaluated by uric acid formation based on spectrophotometry at 293 nm.Citation22 XO activity was expressed in IJ/mg of protein.
Catalase activity
Catalase activity in tissues was determined using slightly modified spectrophotometric method described by Nabavi et al.,Citation23 which is based on the ability of catalase to dissolve the substrate (H2O2), whereby enzymatic reaction is stopped by the addition of ammonium molybdate. Enzyme activity was expressed in μmol/min/mg protein.
The amount of protein in the kidney was determined using the method by Lowry et al.Citation24
Statistical Data Processing
The values of parameters obtained were expressed as X ± SE (mean value ± standard error). Data were analyzed using one-way analysis of variance (ANOVA). The p-value <0.05 was considered statistically significant.
RESULTS
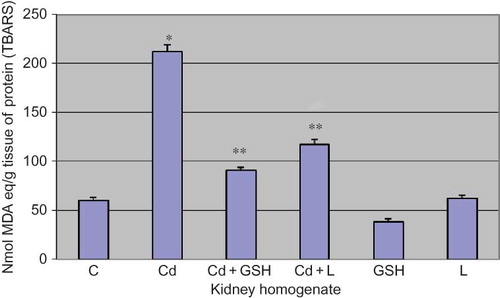
The increased level of TBARS in kidney tissue is statistically significant in cadmium poisoning when compared to control (p < 0.001).
In the group in which GSH was applied alongside cadmium, TBARS concentration was significantly lower when compared to the group poisoned by cadmium (p < 0.001).
In the group in which LA was applied along with cadmium, TBARS concentration was significantly lower when compared to the group poisoned by cadmium (p < 0.001) ().
Figure 2. The level of TBARS in kidney tissue in experimental groups with cadmium, glutathione, and lipoic acid. Data are mean ± SE values. TBARS, thiobarbituric acid-reactive substance (TBARS); C, control group; Cd, cadmium group; MDA, malondialdehyde.
Note: *p < 0.001 versus C, **p < 0.001 versus Cd.
XO activity in the kidney tissue was significantly increased in cadmium poisoning when compared to control (p < 0.001).
In the group in which cadmium and GSH were applied, the activity of XO was reduced when compared to the group poisoned by cadmium (p < 0.001) ().
Figure 3. Xanthine oxidase activity in kidney tissue in experimental groups with cadmium, glutathione, and lipoic acid. Data are mean ± SE values. C, control group; Cd, cadmium group.
Note: *p < 0.001 versus C, **p < 0.001 versus Cd.
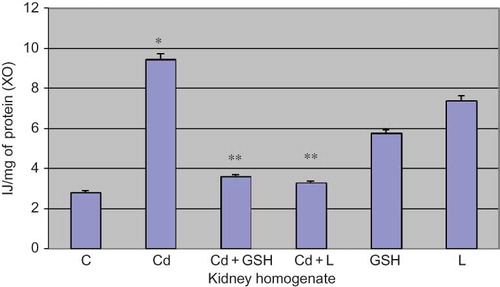
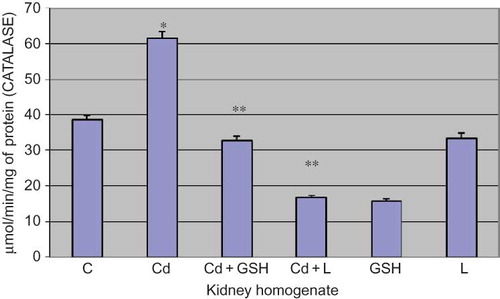
Figure 4. Catalase activity in kidney tissue in experimental groups with cadmium, glutathione, and lipoic acid. Data are mean ± SE values. C, control group; Cd, cadmium group.
Note: *p < 0.001 versus C, **p < 0.001 versus Cd.
In the group in which cadmium and LA were applied, the activity of XO was reduced when compared to the group poisoned by cadmium (p < 0.001).
Catalase activity in the kidney tissue was increased in the group which was administered with cadmium in comparison to control (p < 0.001).
In the group in which cadmium and GSH were administered, the catalase activity was reduced when compared to the group poisoned by cadmium (p < 0.001).
In the group in which cadmium was applied along with LA, the catalase activity was reduced when compared to the group poisoned with cadmium (p < 0.001) ().
DISCUSSION
Cadmium is an industrial pollutant whose concentration in the environment is constantly rising. Therefore, there are growing public concerns regarding the health risks to the industrial workers exposed to cadmium as well as to the people living around industrial zones and the consumers of food and cigarettes polluted by this metal.
Cadmium is taken up from the lung and gastrointestinal tract and transported via the blood to liver and kidney. When exposed to cadmium, both humans and experimental animals may develop renal tubular dysfunction.Citation25 Cadmium induces the synthesis of metallothionein in liver and other tissues. Since proteins of a molecular weight similar to that of metallothionein are completely filtered through the glomerular membrane, CdMT complex is also easily filtered into renal tubular fluid when present in plasma. Subsequent tubular reabsorption of CdMT complex originally described by PiscatorCitation26 is an explanation for the prominent accumulation of cadmium in the kidneys of animals and humans, exposed to cadmium.
In order to test the cadmium poisoning effects, an experimental model was used in which the rats were administered with cadmium in a dose corresponding to the amount of this metal found in human environment. The exposure of rats to cadmium in a dose of up to 50 mg is equivalent to the exposition of people in highly polluted environments.Citation2,27
The cellular processes underlying Cd-induced nephrotoxicity are poorly understood. One of the possible mechanisms of cadmium toxic effect is the one involving oxidative stress. Numerous scientific reports show that substances like fluoride which increases excessive generation of nitric oxide, lipid peroxides and oxygen free radicals; decreases catalase and superoxide dismutase (SOD); reduced GSH; may lead to structural serious damages, especially in the biological membrane structure and functions of the cells and biomacromolecules such as proteins and nucleic acids.Citation28,29 There are many reports that show important role of concomitant oxidative and nitrosative stress in the ensuing renal dysfunction.Citation30 Roles of the hydroxyl radicals have been implicated in the initiation or progression of nephrotoxicity.Citation31
The results of our research show that the level of TBARS (an indicator of lipid peroxidation) is significantly increased in cadmium-poisoned animals. Our results are in accordance with those of Toplan et al.Citation32 and Ognjanović et al.Citation33 who demonstrated an increase in the level of lipid peroxides in rats poisoned by cadmium.
Cd is not a redox-active metal; therefore, the production of ROS by Cd has to be mediated by some indirect mechanism. Although Cd cannot directly participate in Fenton’s reaction, it will indirectly produce ROS by suppressing iron or copper from its normal cell locations which would then enter Fenton’s reaction.Citation8
Oxidative components, such as XO, cytochrome P450 oxidase, and catalase participate in oxidative stress caused by cadmium.Citation34,35 So far, literature data have shown that one of the mechanisms by which cadmium induces the production of ROS is the activation of enzymes that are able to cause oxidative stress.Citation36
The results of our study show that the activity of XO increases in cadmium poisoning, which is in accordance with the findings that XO inhibitor allopurinol and generalized cytochrome P450 oxidase inhibitor ABT inhibit the production of ROS induced by cadmium.Citation37
The effect of this metal on enzyme activity is based on the replacement of metal ions (mostly Zn2+ and Cu2+) in metalloenzymes and the bonding to SH groups (toward which Cd has great affinity) of enzymatic cell systems.Citation10,11
Timbrel et al.Citation11 demonstrated that cadmium inhibits the activity of oxidative enzymes, which is not in accordance with our results. Enzyme inhibition by cadmium may be associated with the separation of essential metal cofactors from the enzymes or with the formation of a covalent bond between cadmium and sulfhydryl and/or other groups, which are responsible for normal enzyme function. Increased activity of XO can be associated with metal–metal interaction, as well as the pro-oxidative activity of cadmium.
Data available in literature show reduced activity of antioxidative enzymes in case of chronic poisoning by cadmium. Jurczuk et al.Citation38 have demonstrated increased catalase and reduced SOD activity in the kidney and liver of rats after chronic cadmium poisoning.
The results of our research show an increase in catalase activity after a 7-day cadmium poisoning, which can be explained as a compensatory measure. The basic role of catalase is decomposition of H2O2 obtained in process of OH radical dismutation.Citation39 Considering that the kidney is an organ involved in the production of free radicals, high concentration of the obtained H2O2 has increased the activity of catalase whose concentration in the kidney tissue is high. GSH and LA have reduced catalase activity because of their antioxidative effect and the reduction of H2O2 concentration.
One of the proposed mechanisms by which Cd can induce the production of free radicals is through the effect on the cellular antioxidative protection system. GSH is considered to be the first line of defense against Cd toxicity because it forms a firm bond with thiol groups.Citation40,41
It has been demonstrated that significant reduction of GSH in the liver increases the hepatotoxicity caused by cadmium.Citation42
A direct consequence of exposure to cadmium in vivo is the exhaustion of physiological antioxidants, such as reduced GSH and proteins containing SH groups, which is proposed as one of the mechanisms of cadmium toxicity.Citation43
Considering that GSH acts as an antioxidant, either directly or via GSH peroxidase/GHS system,Citation43 the results of our research are in accordance with the existing literature data, showing that the application of GSH along with cadmium reduces both the activity of XO and the level of TBARS, probably because of SH group compensation. Active GSH center (–SH) forms a bond with cadmium, thereby reducing its pro-oxidative effect. Role of antioxidants in sodium fluoride-induced toxicity in rat’s kidneys is proven by Nabavi et al.,Citation44 who proved that Curcumin has a potent protective antioxidant effect.
It has been proved that the application of α-LA causes a number of beneficial effects on cellular level, such as direct “trapping” of free oxygen radicals and redox modulation of cellular metabolism. It has the potential to prevent tissue damage induced by the free radicals, whereby the acid itself is oxidized. The basic mechanism of described effects is related to redox systems where LA is converted to dihydrolipoic acid. This is mostly mediated by dihydrolipoamide dehydrogenase. Both NADPH or NADH are coenzymes for liver dihydrolipoamide dehydrogenase, while NADH coenzyme is more commonly present in the heart, brain, and kidneys, even as much as 70–90%. The reduced form of LA is capable of regenerating oxidized GSH, vitamin C, and vitamin E, which are existentially significant in the maintenance of the redox cell status.Citation45,46 Having in mind that literature data refer to the effects of LA in the treatment of nitrosative stress in diabetes, nephropathy, or coronary disease, the obtained findings are significant because they point to the possible application of LA in severe hepatic disorders caused by cadmium. The reduction in XO activity, which is able to produce superoxide anions, as well as the reduction in the level of TBARS as a direct indicator of lipid peroxidation are facts that also point out that LA is capable of ameliorating cadmium poisoning side effects while maintaining the normal metabolic effect of this enzyme, that is, purine base catabolism with the aim of establishing adenylate energy charge within the cell.
CONCLUSION
Based on the results of this research, it can be concluded that the level of lipid peroxide is significantly increased in cadmium poisoning, which can, in part, be ascribed to an increase in the activity of XO. In a 7-day cadmium poisoning, there is a compensatory rise in the activity of catalase as a consequence of increased formation of ROS. GSH and LA, physiological antioxidants administered alongside with cadmium, have reduced the level of lipid peroxide and have also caused the reduction in XO activity, which delineates them as protectors in cadmium poisoning.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Science and Technological development, Republic of Serbia (Projects 43012, 31060, and 41018).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Valko M, Morris H, Metals CM. Toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208.
- WHO. Cadmium. Environmental Health Criteria. Geneva: World Health Organization;1992:134.
- Jarup L. Hazards of heavy metal contamination. Brit Med Bull. 2003;68:167–182.
- Stoeppler E, Weinheim E. Metals and their compounds in the environment. Verlag Chemie. 1991;805–849.
- Jin E, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19(4–5):529–535.
- Piscator M. The nephropathy of chronic cadmium poisoning. In: Foulkes EC, ed. Cadmium. Handbook of Experimental Pharmacology. Vol. 80. New York: Springer; 1986:194.
- Klaassen C, Liu J. Role of metallothionein in cadmium-induced hepatotoxicity and nephrotoxicity. Drug Metab Rev. 1997;29: 79–102.
- Videla L, Fernandez V, Tapia G, Varela P. Oxidative stress-mediated hepatotoxicity of iron and copper: role of Kupffer cells. Biometals. 2003;16:103–111.
- Pavlović D. Slobodni radikali, lipidna peroksidacija i antioksidativna zaštita. In: Koraćević D, Bjelaković G, Đorđević V, Nikolić J, Pavlović D, Kocić G, eds. Biohemija. Beograd: Savremena Administracija; 2006:692–702.
- Jacobson K, Turner J. The interaction of cadmium and certain other metal ions with proteins and nucleic acids. Toxicology. 1980;16(1):1–37.
- Timbrell J. Principles of Biochemical Toxicology. 3rd ed. London: Taylor Francis; 2000.
- Zhang Z, Blake D, Stevens C, Kanczler WJ, Symons M, Benboubetra R. A reappraisal of xanthine dehydrogenase and oxidase in hypoxic reperfusion injury: the role of NADH as an electron donor. Free Radic Res. 1998;28:151–164.
- Sanders S, Massey V. The thermodynamics of xanthine oxidoreductase catalysis. Antioxid Redox Signal. 1999;1:371–379.
- Stohs S, Bagchi D. Oxidative mechanism in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–336.
- Moron M, Depierre J, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S transferase in rat lung and liver. Biochem Biophys Acta. 1979;582:67–68.
- Połeć-Pawlak K, Ruzik R, Lipiec E. Investigation of Cd(II), Pb(II) and Cu(I) complexation by glutathione and its component amino acids by ESI-MS and size exclusion chromatography coupled to ICP-MS and ESI-MS. Talanta. 2007;72:1564–1572.
- Trujillo M, Folkes L, Bartesaghi S, Kalyanaraman B, Wardman P, Radi R. Peroxynitrite-derived carbonate and nitrogen dioxide radicals readily react with lipoic and dihydrolipoic acid. Free Radic Biol Med. 2005;39(2):279–288.
- Rezk B, Haenen G, Van der Vijgh W, Bast A. Lipoic acid protects efficiently only against a specific form of peroxynitrite-induced damage. J Biol Chem. 2004;279(11):9693–9697.
- Liu J, Habeebu S, Liu Y, Klaassen C. Acute CdM KCT injection is not a good model to study chronic Cd nephropathy: comparison of chronic CdCl2 and CdMT exposure with acute CdMT injection in rats. Toxicol Appl Pharmacol. 1998; 153:48–58.
- Sayeda A, Newairy A. Protective role of flax lignans against lead acetate induced oxidative damage and hyperlipidemia in rats. Food Chem Tox. 2009;47:813–818.
- Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012;132:931–935.
- Aygul R, Kotan D, Demirbas F, Ulvi H, Deniz O. Plasma oxidants and antioxidants in acute ischemic stroke. J Int Med Res. 2006;34:413–418.
- Nabavi SF, Nabavi SM, Abolhasani F, Moghaddam AH, Cytoprotective ES. Effects of curcumin on sodium fluoride-induced intoxication in rat erythrocytes. Bull Environ Cont Toxicol. 2012;88:486–490.
- Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193: 265–273.
- Friberg L, Piscator M, Nordberg G, Kjellstrom T. Cadmium in the Environment. 2nd ed. Cleveland, OH: CRC Press; 1974.
- Piscator M. On cadmium in normal human kidneys together with a report on the isolation of metallothionein from livers of cadmium exposed rabbits. Nord Hyg Tidskr. 1964;45:76–82.
- Chalkley S, Richmond J, Barltrop D. Measurement of vitamin D3 metabolites in smelter workers exposed to lead and cadmium. Occup Environ Med. 1998;55:446–452.
- Yu RA, Xia T, Wang AG, Chen XM. Effects of selenium and zinc on renal oxidative stress and apoptosis induced by fluoride in rats. Biomed Environ Sci. 2006;19:439–444.
- Liu GY, Chai CY, Li C. Fluoride causing abnormally elevated serum nitric oxide levels in chicks. Environ Toxicol Pharmacol. 2003;13:199–204.
- Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61:855–861.
- Singh P, Srivastava MM, Khemani LD. Renoprotective effects of Andrographis paniculata (Burm.f.) Nees in rats. Ups J Med Sci. 2009;114:136–139.
- Toplan S, Ozcelik D, Dariyerli N, Akyolcu MC. Oxidant and antioxidant status of cadmium administered rats. J Phys IV. 2003;107:1309–1312.
- Ognjanović B, Pavlović S, Maletić S, . Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol Res. 2003;52(5):563–570.
- Iszard M, Liu J, Klaassen C. Effect of several metallothionein inducers on oxidative stress defense mechanisms in rats. Toxicology. 1995;104:25–33.
- Sarkar S, Yadav P, Bhatnagar D. Lipid peroxidative damage on cadmium exposure and alterations in antioxidant system in rat erythrocytes: a study with relation to time. Biometals. 1998;11:53–57.
- Manca D, Richard A, Van Tra H, Chevalier G. Relation between lipid peroxidation and inflammation in the pulmonary toxicity of cadmium. Arch Toxicol. 1994;68:364–369.
- Sauer J, Waalkes M, Hooser S, Kuester R, McQueen C, Sipes I. Supression of Kupffer cell function prevents cadmium induced hepatocellular necrosis in the male Sprague–Dawley rat. Toxicology. 1997;121:155–164.
- Jurczuk M, Brzoska J, Moniuszko-Jakoniuk M, Gaazyn-Sidorczuk E, Kulikowska-Karpinska E. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol. Food Chem Toxicol. 2004;42:429–438.
- Klaassen C, Bracken W, Dudley R, Goering P, Hazelton G, Hjelle J. Role of sulfhydryls in the hepatotoxicity of organic and metallic compounds. Fundam Appl Toxicol. 1985;5:806–815.
- Chan H, Cherian M. Protective roles of metallothionein and glutathione in hepatotoxicity of cadmium. Toxicology. 1992;72:281–290.
- Dudley R, Klaassen C. Changes in hepatic glutathione concentration modify cadmium-induced hepatotoxicity. Toxicol Appl Pharmacol. 1984;72:530–538.
- Quig D. Cysteine metabolism and metal toxicity. Altern Med Rev. 1998;3:262–270.
- Jian-Ming Y, Arnush M, Qiong-Yu C, Xiang-Dong W, Bing P, Xue-Zhi J. Cadmium-induced damage to primary cultures of rat Leydig cells. Reprod Toxicol. 2003;17:553–560.
- Nabavi SF, Moghaddam AH, Eslami S, Nabavi SM. Protective effects of curcumin against sodium fluoride induced toxicity in rat kidneys. Biol Trace Elem Res. 2012;145:369–374.
- Da Ros R, Assaloni R, Ceriello A. Molecular targets of diabetic vascular complications and potential new drugs. Curr Drug Targets. 2005;6(4):503–509.
- Stoyanovsky D, Tyurina Y, Tyurin A, . Thioredoxin and lipoic acid catalyze the denitrosation of low molecular weight and protein S-nitrosothiols. J Am Chem Soc. 2005;16(45): 15815–15823.