Abstract
Objective: It was found that PAX2 (Paired Box 2) re-expression in renal tubular epithelial cells correlated with renal interstitial fibrosis of rats with obstructive nephropathy. The purpose of the present study was to identify whether RNA interference (RNAi) induced by polyethylenimine (jetPEI) could inhibit PAX2 gene re-expression and impact renal interstitial fibrosis of rats with obstructive nephropathy. Methods: Four pairs of small interfering RNA (siRNA) interference sequences and a negative control were designed and synthesized according to the whole PAX2 gene mRNA sequence. siRNA was then transfected into renal capsule in a rodent model of unilateral ureteral obstruction (UUO), using in vivo jetPEI as delivery vector. The expression of PAX2 mRNA and protein in renal tissue was determined by real-time quantitative polymerase chain reaction (PCR) and Western blot, respectively. A carboxyfluorescein (FAM)-labeled negative control was also synthesized at the same time to identify transfection in vivo. Renal interstitial fibrosis was observed under light microscope after PAX2 gene silenced. Results: Green fluorescence was observed around renal tubule epithelial cells by fluorescence microscopy 3 days after the procedure validating the success of transfection. While the PAX2 gene expression was inhibited by all siRNA interference sequences, the strongest inhibition was observed with siRNA3. The PAX2 mRNA and protein were inhibited by 55% and 81%, respectively. Renal tubular damage and renal interstitial fibrosis were remitted obviously after PAX2 gene silenced. Conclusion: siRNA was able to inhibit the expression of PAX2 in tubular epithelial cells in the UUO model. The siRNA may thus provide therapeutic potential for retarding the pathological progression of UUO.
INTRODUCTION
Obstructive nephropathy is a clinical syndrome resulting from structural and/or functional changes of urinary tract that impede urination, leading to renal parenchymal pathological changes and dysfunction, which is a common cause of chronic kidney disease or end-stage renal disease (ESRD; i.e., renal failure).Citation1 Renal interstitial fibrosis is the final pathway and the major pathological basis of obstructive nephropathy. So far, many experimental investigations, both in vivo and in vitro, support the notion that many factors contribute to the development of renal interstitial fibrosis, including the cell itself, extra cellular matrix, cytokines, growth factors, and interactions between them. Besides, the process is also modulated by many other factors. However, the mechanism of pathogenesis and development of this complicated pathological process is still left unknown. Epithelial-to-mesenchymal transition or transformation (EMT) has been hypothesized to play an important role in this process in recent years.Citation2 From experimental point of view, renal obstruction caused by unilateral ureteral obstruction (UUO) is the most classical model of induced renal fibrosis.Citation3
PAX2 (Paired Box 2) gene that encodes nuclear transcription factor is a key gene to induce mesenchymal-to-epithelial transformation (MET) during embryonic development of kidney. Moreover, it participates in the regulation of embryonic development of kidney at various stagesCitation4 and becomes silenced in mature epithelium of the glomeruli, the proximal tubule, and distal tubule. It was found in recent years that PAX2 gene was re-expressed in renal diseases.Citation5 Cohen et al.Citation6 found that in rat model of UUO, PAX2 gene was re-expressed in collecting ducts and associated with collecting duct cell apoptosis. Our previous work had demonstrated that in rat UUO model, PAX2 gene was indeed re-expressed in tubular epithelial cell, which was positively correlated with the magnitude of renal interstitial fibrosis.Citation7 However, the mechanism by which PAX2 gene acts on EMT and facilitates renal interstitial fibrosis has not been determined yet. This investigation was conducted to explore whether or not the inhibition of PAX2 was associated with the progress of EMT and renal interstitial fibrosis in UUO rats.
RNA interference (RNAi) is a phenomenon induced by double-stranded RNA (dsRNA) in which gene expression is inhibited through specific degradation of mRNA.Citation8 This technique possesses many advantages such as high specificity and high efficiency, as well as low toxicity. Previously small interfering RNA (siRNA) was used to reduce PAX2 protein expression and induce apoptosis in renal cell carcinoma.Citation9 However, whether PAX2 RNAi has any effect on the development of renal fibrosis in UUO has not been investigated yet. Technically, siRNA often has different efficiency at different sites of the same target gene, so the selection and optimization of the target sites are required;Citation10 Furthermore, at least 3–4 pairs of siRNA are usually designed to select the optimized target site. The purpose of the present study was that we used both a surgical UUO model and RNAi technique to design, choose, and identify a siRNA that had the best silencing effect on PAX2 gene, thereby inhibiting its protein expression.
MATERIALS AND METHODS
Materials
A total of 80 male SPF Wistar rats (120–150 g, 4–6 weeks) were obtained from the Laboratory Animal Center of Shengjing Hospital, China Medical University (Shenyang, China). Primary rabbit antibodies to PAX2 was bought from Zymed (South San Francisco, CA, USA); Trizol total RNA extract was from Invitrogen Company; PrimeScriptTMRT Reagent Kit and real-time quantitative PCR (SYBRRPremix Ex TaqTM) reagent box and real-time quantitative PCR Primer were both bought from TaKaRa bioengineering Co. Ltd. Protein lysis buffer was obtained from Shanghai JiKai genetic and chemical technology Company (China). β-Actin primary antibodies and HRP-labeled secondary goat anti-rabbit IgG were both from Santa Cruz (Santa Cruz, CA, USA). Skimmed milk powder was from wondersun dairy stock Co. Ltd. 2′-F modified siRNA was chemically synthesized by JiMa Company (Shanghai, China) and in vivo jetPEI was obtained from PolyPlus Transfection Company.
Methods
Design and synthesization of siRNA
First of all, we got the whole encoding sequences of rat PAX2 gene in NCBI database (Gene ID: 293992, mRNA:NM_001106361). Four pairs of interference target points and random negative control were synthesized by JiMa, Shanghai, China. At the same time, one pair of FAM-labeled negative control was synthesized by siRNA designing software. We also compared the potential siRNA target points sequence with genomic database by BLAST on NCBI to ensure that there was no homology with other genes. Besides, to increase the stability and specificity, the siRNA length of 21 bp was 2′-F modified and two bases were chemically modified in the 3′ top by JiMa Company (Shanghai, China).
Sequences of siRNA
PAX2-siRNA1: sense 5′-CGACUAUGUUCGCCUGG GATT-3′
Anti-sense 5′-UCCCAGGCGAACAUAGUCGGG-3′
PAX2-siRNA2: sense 5′-GUGGGAUCCUACUCCAU CATT-3′
Anti-sense 5′-UGAUGGAGUAGGAUCCCACTG-3′
PAX2-siRNA3: sense 5′-GGAGCGAGUUCUCAGG CAATT-3′
Anti-sense 5′-UUGCCUGAGAACUCGCUCCCA-3′
PAX2-siRNA4: sense 5′-CGAUGUCUUCCAAGCAU CATT-3′
Anti-sense 5′-UGAUGCUUGGAAGACAUCGGG-3′
NC-siRNA sense 5′-UUCUCCGAACGUGUCACGU TT-3′
Anti-sense 5′-ACGUGACACGUUCGGAGAATT-3′
FAM-siRNA sense 5′-UUCUCCGAACGUGUCACG UTT-3′
Anti-sense 5′-ACGUGACACGUUCGGAGAATT-3′
Animal Model Preparation and Identification
Model preparation
Wistar rats were randomly divided into two groups: sham-operated group (sham group) and UUO group, 10 in each group. Rats in UUO group were anesthetized by intraperitoneal injection of 10% chloral hydrate (300 mg/kg); the left kidney and ureter were exposed via a flank incision. Left ureter was identified along the inferior pole of left kidney, which was then ligated at both proximal and distal points using 4–0 silk suture. Ureter was severed between these two ligation points to prevent retroinfection. In sham group, ureter was isolated but not ligated. The incision was closed in every single layer. After the surgery, rats were sacrificed at the third day, to remove and collect the left kidney. A small portion of the renal tissue was taken and fixed in 4% paraformaldehyde for pathomorphology assays, then embedded in paraffin for following pathomorphology observation. Principles of laboratory animal care were followed in the experiment.
Pathomorphology observation and evaluation
Renal tissue was embedded in paraffin and stained with standard HE method. Under light microscopy, tubulointerstitial damage was assessed.Citation11 The pathologist, who read and scored the tissue damage, was unaware of the group assignment of individual rat.
Fibrosis was assessed by measuring collagen deposition in Masson’s trichrome-stained slides according to the standard protocol.Citation12 In each section with Masson’s trichrome staining, 10 fields were digitized and scanned at 400× magnification by using NIS-Elements BR 2.10 image analysis software (Nikon, Tokyo, Japan). Using a grid superimposed on the image, the number and percentage of blue collagen area in the tubulointerstitial region were measured. Average of this ratio represented relative area of renal interstitium on each slide.
Transfection in vivo
PAX2-siRNA (four silent points and FAM-labeled and unlabeled negative control) and in vivo jetPEITM(N/P = 5) complex was confected strictly according to the instruction, and the final volume was 200 μL.
In vivo jetPEI was purchased from PolyPlus Transfec-tion (Illkirch, France). To generate PAX2-siRNA-PEI (FAM-labeled and unlabeled negative comparison siRNA and four silent points siRNA), 80 μg of siRNA specific for the rat PAX2 mRNA were complexed with in vivo jetPEI at an N/P ratio of 5, following the recommendations of the manufacturer (PolyPlus Transfection).
When the UUO model was completed, 200 μL PAX2-siRNA-in vivo jetPEI solution was injected into renal capsule at multi-points. Then the rats were divided into five groups based on different siRNA points, eight in each group. At the third day after injection, rats were sacrificed and the left kidneys were taken out for dissecting renal cortex from medulla for the following experiments.
Identification of PAX2-siRNA-PEI transfection in kidney
Three days after the renal transfection of PAX2-FAM-siRNA-PEI or PAX2-NC-siRNA-PEI, the left kidneys of rats were taken out, embedded in OTC for freezing section (5 μm in thickness) preparation (freezing microtome; Leica, Solms, Germany), then used fluorescence microscopy (Olympus, Tokyo, Japan) to observe distribution of green fluorescence in renal tissue.
Real-time quantitative PCR to analyze PAX2 mRNA expression
Total RNA was extracted from renal tissue using TRIzol (Invitrogen, Carlsbad, CA, USA), and the operation procedure were the same as our previous description.Citation7 The primers for PAX2 and internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were as follows: PAX2, sense 5′-CGG TGAGAAGAGGAAAC GAG-3′ and antisense 5′-GCTTGGAAGACATCGG GATA-3′; GAPDH, sense primer 5′-TGTGTCCGTCGTGGATCTGA-3′ and antisense primer 5′-ATGGTGGTGAAGACGCCAGTA-3′. Serial dilutions of an arbitrary cDNA pool were used to establish a standard curve. All samples were run in triplicates. The threshold cycle (Ct) values were determined by ABI 7500 Software (Applied Biosystems, Foster City, CA, USA) and normalized by subtracting the Ct GAPDH values. The relative amount of mRNA of each sample was calculated using the 2-ΔΔCt method in individual experiments.Citation13
Western blot to analyze PAX2 protein expression
Proteins were isolated from the phenol-ethanol supernatant, and Western blotting was performed as described previously.Citation7 Semi-quantitative method was used to analyze PAX2 expression in renal tissue and protein bands on membrane were scanned and saved in the computer. Gel imaging software system was used to analyze the optical density of protein bands and results were normalized to β-actin level.
Pathological changes of renal interstitial fibrosis after PAX2 gene silencing
With the UUO model completed, 20 rats was divided into negative control group and silence group, 10 in each group. PAX2-siRNA-in vivo jetPEI solution was injected (optimal point screened by real-time quantitative PCR and Western blot or negative control) into the renal capsule of the two groups, respectively. Fourteen days after the injection, rats were killed and the left kidneys were taken out for the following pathomorphology observation.
Statistical analysis
All data are presented as means ± standard deviation (SD). Student’s t-test or one-way analysis of variance (ANOVA) was performed with SPSS version 13.0 for windows (SPSS Science Inc., Chicago, IL, USA) whenever appropriate. Probability ( p) of less than 0.05 was considered significant.
RESULTS
Morphological Changes of Renal Tissue
Pathological changes of kidney under light microscope
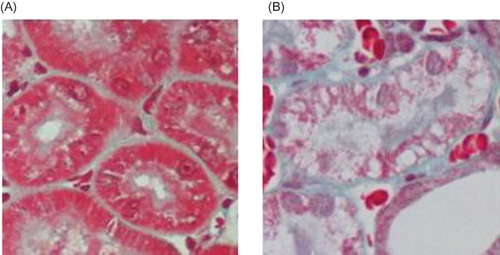
Renal interstitial edema, tubular dilatation inflammatory cells infiltration were observed under the light microscope in UUO group, whereas such changes were not observed in Sham group. However, no visible glomerular lesions were detected in both the groups. There were significant differences in renal tubular damage scores between the sham and the UUO groups, p < 0.05. ( and ).
Figure 1. HE staining. (A) No abnormal changes were detected in the Sham group. (B) In the UUO group, tubular edema appeared and inflammatory cell infiltrated in renal interstitium.
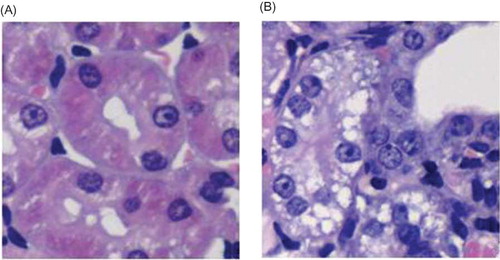
Table 1. Renal tubular injury score and relative area of renal interstitium in each group (%, mean ± SEM n = 10).
Changes of renal interstitial fibrosis
Masson staining showed that, in sham group, renal collagen staining mainly located at tubular basement membrane, renal capsule, mesangial region, and area surrounding capillaries between renal tubules. Little staining was found around interstitium between renal tubules. In UUO group, collagen deposition was visible, and renal interstitial fibrosis relative area was significantly larger than that of the sham group ( p < 0.05). ( and ).
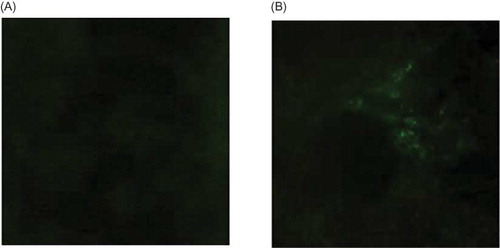
Transfection of PAX2-siRNA-PEI in Renal Tissue
The kidneys were taken out and frozen sections were prepared 3 days after the injection of FAM-siRNA-PEI and NC-siRNA-PEI. Then the frozen sections were observed under fluorescence microcopy. Green fluorescence was found around tubule in kidney injected FAM-siRNA-PEI (B), while nothing in kidney injected NC-siRNA-PEI (A).
Real-Time PCR Analysis of PAX2-siRNA-PEI Inhibition on PAX2 mRNA Expression
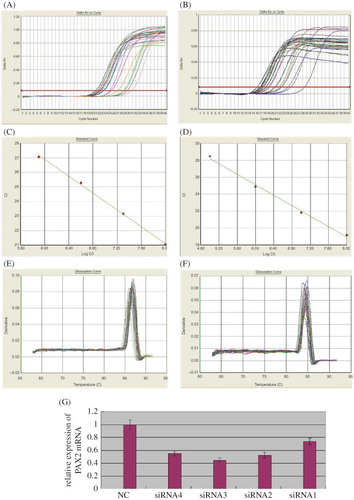
On the third day after transfection, cortex was taken for real-time PCR. PAX2 and GAPDH dissociation curves had the same solution temperature, which indicated that the product was specific without nonspecific ones and primer. Through the calculation of the CT number got from amplification curve and standard curve with 2−ΔΔCT method, the inhibiting effects of the four pairs of PAX2-siRNA-PEI on the expression of PAX2 mRNA in renal cortical were got, separately siRNA1 26%, siRNA2 48%, siRNA3 55%, and siRNA4 45%. PAX2-siRNA3 had the optimal inhibiting effect, which indicated that PAX2-siRNA3 was the optimal point to inhibit PAX2mRNA expression ().
Figure 4. Real-time quantitative PCR analysis of PAX2 mRNA expression. (A) PAX2 amplification curve. (B) GAPDH amplification curve. (C) PAX2 standard curve, Ct = −2.884167 × Log10 C0 + 44.182236 (C0: the starting copy number). (D) GAPDH standard curve, Ct = −3.088276 × Log10 C0 + 43.657402 (C0: the starting copy number). (E) PAX2 dissociation curve, solution temperature is 87°C. (F) GAPDH dissociation curve, solution temperature is 84°C. (G) mRNA transcription of PAX2 in UUO assessed by real-time PCR after PAX2-siRNA-PEI was transfected. The normalized PAX2 mRNA level of negative control-siRNA-PEI was taken as 1.0.
Western Blotting Analysis of PAX2-siRNA-PEI Inhibition on PAX2 Protein Expression
According to the results of immunotracing method, a band was observed at 43 and 46 kDa on each of the five electrophoresis tracks, which was coordinated with the specific protein bands β-actin and PAX2 protein products. β-Actin bands had similar brightness, while PAX2 bands were different in brightness. Compared with the control group, the protein expression of PAX2 in siRNA3 group decreased by 81%, which was coordinated with the results of real-time PCR analysis. ()
Figure 5. Western blotting analysis of PAX2 protein expression. (A) Expression of PAX2 and β-actin proteins in each group transfected by different PAX2-siRNAs; (B) protein of PAX2 in UUO assessed by Western blotting 3 days after PAX2-siRNA-in vivo jetPEI was transfected. The normalized PAX2 protein level of negative control-siRNA-in vivo jetPEI was taken as 1.0.
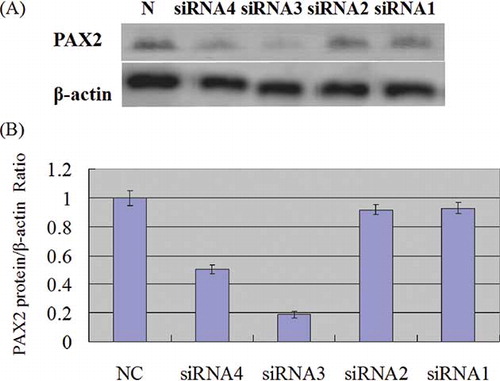
Figure 6. Gross anatomy of kidney. (A) Left kidney of negative control group was significantly enlarged. (B) left kidney of silence group was significantly smaller than that of control group. (C) Kidneys in negative control group showed obviously thinner renal parenchyma, dilated renal pelvis, compressed papilla and flattened and blunt fornix papilla. (D) Left kidney of silence group appeared thicker renal parenchyma, alleviated renal pelvis dilation in contrast with that of negative control group (the upper organ is right kidney in silence group, the lower one is left kidney in silence group).
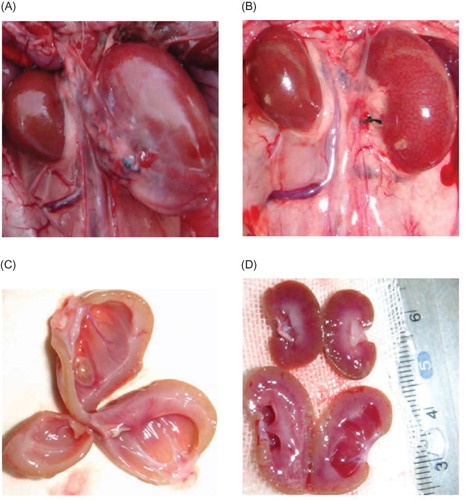
Figure 7. HE staining. (A) In negative control group, renal tubular structure was severely damaged, that is, collapsed lumen, diffusive infiltration of fibroblast in renal interstitium, and collagen formation. (B) In contrast, kidney in silence group appeared alleviated dilation of renal tubules, thinner renal interstitium, and less interstitial collagen deposition.
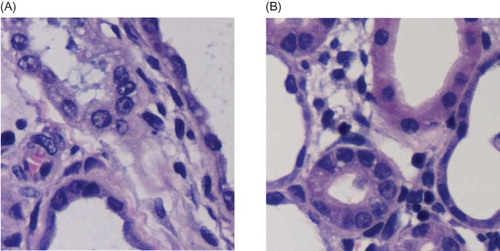
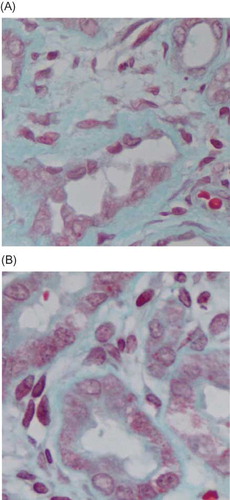
Figure 8. Masson staining (blue represents the collagen deposition). (A) In negative control group, renal interstitial fibrosis significantly increased, with renal interstitium widening and obvious collagen deposition. (B) In silence group, interstitial fibrosis was slighter with less collagen deposition. Original magnification 400×.
Morphological Changes of Renal Tissue after PAX2 Gene Silencing
Macroscopic observation
Rat’s left kidney of negative control group presented some changes, such as visible hydronephrosis, thinner renal parenchyma, dilated renal pelvis, compressed papilla, and flat and blunt fornix papilla, some of which became concave shape. On the contrary, kidneys of silence group did not exhibit overt morphological alterations. ()
Pathological changes of kidney under light microscope
HE staining showed that, in rats from negative control group, renal tubular structure was severely damaged. Renal tubules became atrophic or dilated into cystic shape. Tubular lumen collapsed and a large number of epithelial cells underwent necrosis. Diffusive infiltration of macrophage and lymphocyte appeared at renal interstitium, with obvious fibroblast hyperplasia and interstitial collagen deposition. Such significant changes in the histological appearance from silence group were not observed. There were significant differences in renal tubular damage scores between the negative control and silence groups (p < 0.05). ( and ).
Table 2. Renal tubular injury score and relative area of renal interstitium in each group (%, mean ± SEM n = 10).
Changes of renal interstitial fibrosis
Masson staining showed, in negative control group, renal interstitial fibrosis significantly increased, with increased extracellular matrix complexity and collagen deposition. Compared with the control group, the changes from the silence group such as the dilation of renal tubules, thickness of renal interstitium and deposition of interstitial collagen were slight. Renal interstitial fibrosis relative area was lower than that of the negative control group (p < 0.05) ( and ).
DISCUSSION
RNAi blocks the expression of certain gene with high efficiency, thus leading to silencing posttranscription through intracellular-specific induction of the homogeneous complementary double-stranded RNA, which degrades mRNA.Citation14 As a new gene-blocking technique, RNAi has become an important tool of gene function research, with the advantages of high specificity, high efficiency, high stability, and lower toxicity.Citation15 According to previous report,Citation16 siRNA, which is 21-bp long with two 3′-overhangs can silence mammal gene effectively and specifically. The chemical synthesis of siRNA in vitro is the oldest and most traditional way of synthesis, which has the advantage of easy preparation, high purity and low toxicity, easy labeling, and tracking in vivo. However, unmodified or naked siRNA is with a short half-time and not able to maintain an effective concentration in circulation. Therefore, it is necessary to modify the siRNA, which can increase its stability and bioavailability,Citation17 make it resistant to RNase in blood stream,Citation18 and decrease side effects and “off target” effect.Citation19 In the present study, the PAX2-siRNA with 21 bp that was two 3′-overhanged and 2′-F modified was chosen and chemically synthesized, which can not only strengthen the stability of siRNA in vivo but also improve the specificity and the interferential effect in mammalian cell.
RNAi has been widely applied to research into mammalian cells in vitro, but the transfection of siRNA into mammals or human body remains very challenging. One of the difficulties is how to deliver the siRNA to specific target organs or cells safely and effectively.Citation20 At present, there are mainly two types of vectors used for genetic therapy: viral vector and nonviral vector.Citation21 Virus vectors include adenovirus vector, adeno-associated virus, retroviral vector, lentiviral vector, and so on. Viral vectors have potential toxicity, For example, immunogenicity can trigger certain degree of immune reaction;Citation22 virus gene products will have certain toxicity to the host cells; and virus gene group may integrate with the host genes randomly causing the abnormal expression of the host genes.Citation23 Moreover, it is complicated to synthesize virus vector and apply repetitively in vivo. In order to avoid the disadvantages of viral vector use, nonviral vectors are investigated alternatively.Citation24 Compared with viral vectors, nonviral vectors have such advantages as high stability and safety, easy to construct, and produce on a large scale without infection and immunogenicity. Among the nonviral vectors, PEI with a high rate of transfection is considered as an effective tool, both in vivo and in vitro,Citation25,26 which has been widely used in recent years.Citation27,28,29
In the present experiment, in vivo jetPEI transfection solution was combined with chemically modified siRNA to increase the rate of transfection. The combination was labeled with FAM and injected into the subcapsule of the kidney in rats with UUO. Green fluorescence was observed under the fluorescent microscope, indicating that the PAX2-siRNA-PEI complex injected into subcapsule had entered renal parenchyma. In other words, RNAi was introduced to the targeted kidney.
UUO is a most traditional model for renal fibrosis induction because of its simple surgical procedure, reproducibility, and rapid onset of renal fibrosis (appeared on the third day after the obstruction).Citation3 In the present study, diffused macrophages and lymphocytes infiltration in renal mesenchyma, renal tubule expansion, renal mesenchyma widening, and collagen fiber deposition were all observed, which depicted renal fibrosis after UUO.
At present, siRNA is usually designed by software or obtained from already existing data-bank. To our knowledge, the application of RNAi for PAX2 in rats has not yet been reported. Because the gene silence effect is greatly reduced by mismatched sequence and position by one or two base pair,Citation30 at least three to five pairs of candidate sequence should be designed. Then the optimal target point should be screened through parallel experiments when there is no unquestionable siRNA “gene base,” in order to ensure the effective and specific silencing potency. Four pairs of PAX2-siRNA were synthesized in the present study and after 2′-F modification they were transfected into the subcapsule of the kidney using vivo jetPEI as delivery vector. The inhibition capability of the four siRNA to PAX2 mRNA expression was tested by the real-time PCR. Among all siRNA interference sequences on PAX2 gene expression, siRNA3 site revealed the strongest inhibition effect. PAX2 protein expression measured by Western blotting showed that protein expression on each point was inhibited and PAX2-siRNA3 silencing point had the lowest protein expression. When the expression of PAX2 gene was inhibited by siRNA3, the renal tubules damage and interstitial fibrosis were relieved compared with that in the control group. This result suggests that the PAX2 gene silencing can reduce the damage of renal tubules and delay the interstitial fibrosis, therefore there may be a therapeutic effect on renal interstitial fibrosis.
In summary, the present study demonstrated that the expression of PAX2 was inhibited by siRNA delivered with jetPEI in UUO model in vivo. Among four pairs of siRNA designed for PAX2, siRNA3 was with strongest inhibitory efficacy, therefore providing a new tool to explore the potential role of PAX2 gene in the pathogenesis of renal interstitial fibrosis and to specifically target PAX2 gene expression for experimental therapeutics.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported in part by a scientific and technological development grant from Shenyang municipality (F10-205-1-29) and partly supported by scientific and technological research grant from Jiamusi University (Sz2011–011).
REFERENCES
- Chen F. Genetic and developmental basis for urinary tract obstruction. Pediatr Nephrol. 2009;24(9):1621–1632.
- Chevalier RL, Thornhill BA, Forbes MS, Kiley SC. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol. 2010; 25(4):687–697.
- Picard N, Baum O, Vogetseder A, Kaissling B, Le Hir M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem Cell Biol. 2008;130(1):141–155.
- Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. PAX2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol. 2007;18(4):1121–1129.
- Lindoso RS, Verdoorn KS, Einicker-Lamas M. Renal recovery after injury: The role of Pax-2. Nephrol Dial Transplant. 2009;24(9):2628–2633.
- Cohen T, Loutochin O, Amin M, Capolicchio JP, Goodyer P, Jednak R. PAX2 is reactivated in urinary tract obstruction and partially protects collecting duct cells from programmed cell death. Am J Physiol Renal Physiol. 2007;292(4):F1267–F1273.
- Li L, Wu Y, Zhang W. PAX2 re-expression in renal tubular epithelial cells and correlation with renal interstitial fibrosis of rats with obstructive nephropathy. Ren Fail. 2010;32(5): 603–611.
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669): 806–811.
- Hueber PA, Waters P, Clark P, Eccles M, Goodyer P. PAX2 inactivation enhances cisplatin-induced apoptosis in renal carcinoma cells. Kidney Int. 2006;69(7):1139–1145.
- Qing L, Quan K, Song W, . Selection and validation of optimal siRNA target sites for RNAi-mediated gene silencing. Gene. 2007;395(1–2):160–169.
- Taal MW, Zandi-Nejad K, Weening B, . Proinflammatory gene expression and macrophage recruitment in the rat remnant kidney. Kidney Int. 2000;58(4):1664–1676.
- Mizuguchi Y, Miyajima A, Kosaka T, Asano T, Asano T, Hayakawa M. Atrovastatin ameliorates renal tissue damage in unilateral ureteral obstruction. J Urol. 2004;172(6 Pt 1): 2456–2459.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T)method. Nat Protoc. 2008;3(6):1101–1108.
- Moffat J, Sabatini DM. Building mammalian signalling pathways with RNAi screens. Nat Rev Mol Cell Biol. 2006;7(3):177–187.
- Kumar LD, Clarke AR. Gene manipulation through the use of small interfering RNA (siRNA): From in vitro to in vivo applications. Adv Drug Deliver Rev. 2007;59(2–3):87–100.
- Wilson R, Purcell D, Netter HJ, Revill PA. Does RNA interference provide new hope for control of chronic hepatitis B infection? Antivir Ther. 2009;14(7):879–889.
- Bramsen JB, Laursen MB, Nielsen AF, . A large-scale chemical modification screen identifies design rules to generate siRNAs with high activity, high stability and low toxicity. Nucleic Acids Res. 2009;37(9):2867–2881.
- Ui-Tei K, Naito Y, Zenno S, . Functional dissection of siRNA sequence by systematic DNA substitution: Modified siRNA with a DNA seed arm is a powerful tool for mammalian gene silencing with significantly reduced off-target effect. Nucleic Acids Res. 2008;36(7):2136–2151.
- Choung S, Kim YJ, Kim S, Park HO, Choi YC. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem Biophys Res Commun. 2006;342(3):919–927.
- Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: Challenges and future directions. Nat Rev Cancer. 2011;11(1):59–67.
- Duan Y, Zhang S, Wang B, Yang B, Zhi D. The biological routes of gene delivery mediated by lipid-based non-viral vectors. Expert Opin Drug Deliv. 2009;6(12):1351–1361.
- Zaiss AK, Muruve DA. Immune responses to adeno-associated virus vectors. Curr Gene Ther. 2005;5(3):323–331.
- Karmali PP, Chaudhuri A. Cationic liposomes as non-viral carriers of gene medicines: Resolved issues, open questions, and future promises. Med Res Rev. 2007;27(5):696–722.
- Behlke MA. Progress towards in vivo use of siRNAs. Mol Ther. 2006;13(4):644–670.
- Nimesh S, Chandra R. Polyethylenimine nanoparticles as an efficient in vitro siRNA delivery system. Eur J Pharm Biopharm. 2009;73(1):43–49.
- Jere D, Jiang HL, Arote R, . Degradable polyethylenimines as DNA and small interfering RNA carriers. Expert Opin Drug Deliv. 2009;6(8):827–834.
- Hassani Z, Francois JC, Alfama G, . A hybrid CMV-H1 construct improves efficiency of PEI-delivered shRNA in the mouse brain. Nucleic Acids Res. 2007;35(9):e65.
- Liao HW, Yau KW. In vivo gene delivery in the retina using poly- ethyleneimine. Biotechniques. 2007;42(3):285–6, 288.
- Bolcato-Bellemin AL, Bonnet ME, Creusat G, Erbacher P, Behr JP. Sticky overhangs enhance siRNA-mediated gene silencing. Proc Natl Acad Sci USA. 2007;104(41):16050–16055.
- Yoshinari K, Miyagishi M, Taira K. Effects on RNAi of the tight structure, sequence and position of the targeted region. Nucleic Acids Res. 2004;32(2):691–699.