Abstract
Aim: The purpose of this study was to determine the effect of mesenchymal stem cell (MSC) transplantation on the peritoneal morphology and inflammation markers in rat models of peritoneal dialysis (PD). Materials and methods: Wistar albino rats were divided into two groups: control (C) (n = 8) and experimental groups (n = 50). PD solution was given to the experimental group during 6 weeks. Then, experimental group was divided into three groups as PD, MSC, and placebo (P) groups. MSC group was treated with MSC (1.5 × 106 cells/kg) and P group was treated with phosphate buffer solution via intraperitoneal injection. Evaluation was performed to C and PD groups at the end of 6 weeks and to MSC and P groups at second and third week of the treatment (MSC-2, P-2, MSC-3, and P-3 groups). Results: The submesothelial area was significantly thickened in PD and P groups compared to C and MSC groups. Peritoneal fibrosis was seen in P-3 group but not in MSC group. There were no significant differences between the MSC-3 and C groups according to morphological findings. Levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were significantly increased in MSC-2 group compared to the other groups (p-values ranged from 0.0001 to 0.04). TNF-α and IL-6 levels in MSC-3 and P-3 groups were lower than PD and C groups (p < 0.0001 for TNF-α and p = 0.0001–0.002 for IL-6). Conclusion: Giving MSC may protect the peritoneal membrane from the deleterious effect of PD and extend the life of the peritoneal membrane. Our study is the first on this issue and more detailed studies are needed.
INTRODUCTION
Peritoneal dialysis (PD) is the first preferred dialysis modality for children with end-stage renal disease, especially during early childhood and infancy. The most important challenge for PD is that it is not possible to preserve peritoneal membrane integrity for a long term. The peritoneal membrane is progressively damaged during the chronic PD because of continuous exposure to unphysiologic dialysis solutions, leading to chronic inflammation. When the peritoneal membrane is injured, inflammatory cytokines such as interleukin- (IL-) 1, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) and growth factors such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and fibroblast growth factor-2 may be produced by mesothelial cells and macrophages. Chronic PD is associated with morphologic changes in the peritoneal membrane, including loss of mesothelial cells, fibrosis of the submesothelial layer, neoangiogenesis, vasculopathy, and basement membrane thickening of mesothelial cells and stromal blood vessels. All these lead to ultrafiltration failure.Citation1–4 At 2–6 years, ultrafiltration failure occurs in about 30% of PD patients.Citation5
Mesenchymal stem cells (MSCs) are self-renewing with a capacity to differentiate into several different cell types from all three germ layers. These cells have attracted significant attention as a potential therapeutic agent for tissue repair due to their ability to migrate to the damaged tissue.Citation6,7 Recent experimental studies have showed that MSC can efficiently attenuate inflammatory processes, for instance, myocardial infarctionCitation8 and acute lung injury.Citation9 It was shown that MSC transplantation in a rat model of myocardial infarction decreased the expression of inflammatory cytokines such as TNF-α, IL-1β, and IL-6.Citation8 In an acute lung injury model, it was observed that MSC therapy reduced the levels of pro-inflammatory cytokines, increased the level of anti-inflammatory cytokines in the plasma and bronchoalveolar lavage.Citation9
We aimed to determine the effect of MSC transplantation on the peritoneal morphology and inflammation markers in rat models of PD.
MATERIALS AND METHODS
Animals and Ethical Aspects
In this study, adult male Wistar albino rats weighing 341 ± 35 g were used. The animals were allowed a standard diet and water ad libitum. The experiment was reviewed and approved by the local ethical committee on the use of laboratory animals of the Erciyes University.
Experimental Design
Study design was shown in . Fifty-eight Wistar albino male rats were divided into two groups: experimental group (n = 50) and control (C) group (n = 8). Rats without dialysis and treatment were used as a control group. Experimental group was dialyzed with commercial dialysis solution, containing 3.86% glucose, once daily for 6 weeks. Then, at the end of 6 weeks, the experimental group was divided into three groups: PD, MSC, and placebo (P) groups. C and PD groups were sacrificed and evaluated at the end of 6 weeks. MSC group was treated with MSC in phosphate buffer solution (PBS), and P group was treated with PBS as a placebo via intraperitoneal injection. P and MSC groups were evaluated at the second and third week of MSC/PBS treatment (MSC-2, P-2, MSC-3, and P-3 groups). The samples of parietal peritoneum were taken from all rats, and then the rats were sacrificed.
Experimental Peritoneal Dialysis Model
The PD solution was administered to the rats in the experimental group by direct intraperitoneal injection with a syringe and 22-G needle for 6 weeks. The left and right lower quadrants were used alternately for the daily injections. Prophylactic antibiotic (ceftazidime, 125 mg/L) was added to dialysis solution. Instilled volume was gradually increased from 10 mL to 20 mL during the first 3 days.
Mesenchymal Stem Cells
Frozen vials of green fluorescent protein- (GFP-) labeled purified MSC from bone marrow of the rats were purchased from Kocaeli University Center for Stem Cell and Gene Therapies Research and Practice (Kocaeli, Turkey). These stem cells were stored at –80°C until they are used. The frozen vial of cells was taken from the refrigerator and rapidly thawed in a 37°C water bath for 1 min. Promptly, vial was disinfected with 70% ethyl alcohol and opened. The cells were resuspended by gently pipetting, and they were transferred to a sterile 15-mL conical tube. A total of 5–6 mL of medium (100 mL medium content = 88 mL of Roswell Park Memorial Institute medium, 10 mL of fetal bovine serum, 1 mL of l-glutamine, and 1 mL of penicillin–streptomycin solution) was added into the tube. The suspended cells were centrifuged at 800 rpm for 5 min. The supernatant was discarded. A total of 3–4 mL of medium was added and pipetting was made. The suspended cells were again centrifuged. The pellet was suspended in 2 mL of medium, and then propagated in a culture flask. The flask was placed in 37°C, 5% CO2 humidified incubator. The next day, fresh medium was changed by the washing process with PBS. Then, medium was changed every 3 days until the cells reach 70–80% confluent. The medium was discarded, the cultures were washed with PBS, and the cells were harvested using 2 mL of 0.25% trypsin. After centrifugation and pipetting process, cells were again inoculated in flask. After trypsinization, cells were washed twice with PBS, and the third passage was used. Cells were administered by intraperitoneal injections of 1.5 × 106 cells/kg/rat.
Peritoneal Tissue Sampling
The animals were anesthetized with ketamine (80 mg/kg) and xylazine (40 mg/kg). The peritoneal cavity was opened. Samples of parietal peritoneum at the upper portion with and without muscle tissue were taken for examination. Then, the animals were sacrificed.
MORPHOLOGICAL ANALYSIS
Light Microscopy
Parietal peritoneum with muscle tissue was fixed in 4% neutral buffered formalin and embedded in paraffin. Five-micrometer sections were cut for histological examination. The samples were stained with hematoxylin and eosin (H&E) stain. All samples were examined qualitatively whether there was inflammation and neovascularization in a blinded fashion. Thickness of the submesothelial extracellular matrix was determined. For determination of thickness of the submesothelial extracellular matrix, 14 independent measurements were taken and averaged for each animal. Thickness was expressed in micrometers.
Immunofluorescence Microscopy
Immunofluorescent microscopy was used to show whether intraperitoneally given GFP-labeled MSC adhered to peritoneum. Tissue taken from the parietal peritoneum was embedded in tissue freezing medium and was immediately frozen at –20°C. After freezing, tissues were transferred to lysine glasses by taking 7-μm sections in frozen microtome. Direct aspects and praeparatum were evaluated by using blue light in an Olympus BX50 branded immunofluorescence microscope (Olympus, Tokyo, Japan). Praeparatum displaying green fluorescence along the peritoneal line were regarded as being positively stained. The digital images were captured and recorded using an Olympus E330 digital camera (Olympus).
Measurement of TNF-α, IL-6, VEGF, and TGF-β Content in Peritoneal Tissues
Samples of parietal peritoneum without muscle tissue were used for the measurement of TNF-α, IL-6, VEGF, and TGF-β levels. Wet peritoneal weight was recorded. Tissues were placed in PBS. The weight/volume ratio was 1 g wet tissue/7 mL of PBS. Tissues were then homogenized with a mechanical tipped homogenizer and centrifuged at 10,000 × g for 30 min at 4°C. Supernatants were stored at –80°C until analysis. TNF-α, IL-6, VEGF, and TGF-β levels in the supernatants were assayed by enzyme-linked immunosorbent assay (ELISA) using commercial kits [Rat TNF-α ELISA kit (Invitrogen, Renfrewshire, UK), rat IL-6 Platinum ELISA kit (eBioscience, Vienna, Austria), Rat VEGF ELISA kit (RayBio®, Norcross, GA, USA), and Multispecies TGF-β1 kit (Biosource, Nivelles, Belgium)] according to the manufacturer’s instructions. Results were expressed as pg/g wet weight of tissue for VEGF and TNF-α and pg/mg wet weight of tissue for IL-6 and TGF-β1.
Statistical Analysis
Statistical analysis was performed using Version 17.0 of SPSS for Windows. First, the distributions of all data were determined by using the Shapiro–Wilk test. The data with normal distribution were expressed as mean ± SD, and the data with non-normal distribution were expressed as median (min–max). The comparisons between the groups for the data with normal distribution were done using ANOVA with the post hoc Tukey procedure and for the data with abnormal distribution were done using the Kruskal–Wallis test. Then, when a statistical significance was found in Kruskal–Wallis test, differences between two groups were tested by the Mann–Whitney U-test. Comparison of the histopathological findings was done by the chi-square test. All tests were considered as statistically significant if p-values were <0.05.
RESULTS
Droup-Out
In the first 6 weeks, a total of six rats died in experimental group. In the last evaluation, the distribution of rats was as follows: eight rats in C, eight rats in PD, nine rats in MSC-2, nine rats in P-2, nine rats in MSC-3, and nine rats in P-3 groups.
Implantation of MSC
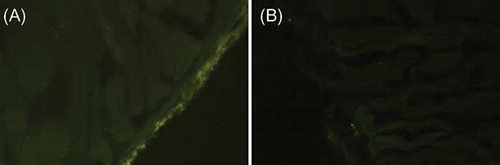
The presence of GFP-labeled MSC was detected in the parietal peritoneum by immunofluorescent microscopy. Under immunofluorescent microscopy, GFP-labeled MSCs were visible by fluorescence excitation in the mesothelium line of MSC groups. No fluorescence excitation was seen in the P groups ().
Histological Changes and the Submesothelial Thickness
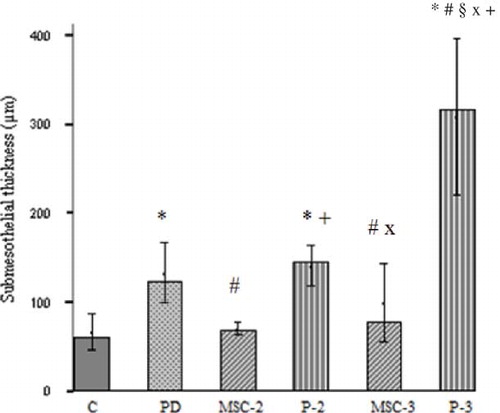
Exposure of the dialysis solution for the 6 weeks resulted in inflammation (p = 0.03, PD group vs. C group), and an increase of the submesothelial thickness was an indicator of fibrosis (p = 0.001, PD group vs. C group) in the parietal peritoneum. More neovascularization processes were observed in the PD group than in the C group, although this difference was not reached to statistically significant levels (p = 0.06). In MSC-2 group, although increase in inflammation had borderline significance (p = 0.053), neovascularization process did not significantly increase (p = 0.08) when compared with C group. There were no statistical differences between C with P-2 and MSC-3 groups in terms of inflammation and neovascularization. Submesothelial thickness increased in P-2 group compared with C group (p < 0.0001). In the MSC-3 group, submesothelial thickness tended to increase compared with the C group but not significant (p = 0.07). Neovascularization and submesothelial thickness were significantly increased in P-3 group compared to C group (p = 0.03 and p < 0.0001, respectively). No significant differences were found between MSC-2 and P-2 groups in terms of inflammation and neovascularization, but submesothelial thickness significantly decreased in MSC-2 group compared to P-2 group (p < 0.0001). Remarkable increase of submesothelial thickness was observed in the P-3 group compared with that in the MSC-3 group (p < 0.0001). Furthermore, submesothelial thickness significantly increased in P-3 group compared with P-2 group (p = 0.004). In MSC-3 group, submesothelial thickness was not different from MSC-2 (p = 0.2) ( and ).
Figure 3. H&E staining of representative samples of peritoneal tissues. (A) Control rat peritoneum. (B) In PD group, there was thickening of the submesothelial area with cell infiltration and neoangiogenesis. (C) In the MSC-2 group, there was similar submesothelial thickness compared to C group, and cellularity was increased. (D) In the P-2 group, submesothelial thickness increased with cellularity. (E) In the MSC-3 group, morphological changes did not significantly differ in C group. (F) In the P-3 group, marked peritoneal fibrosis with neovascularization was seen. Scale bars, 200 μm.
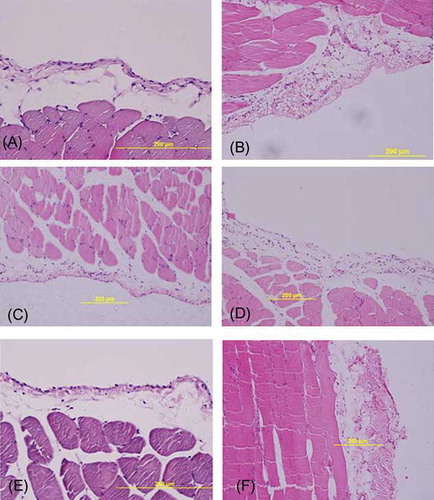
Figure 4. Submesothelial thickness (μm). The most significant change in thickness was observed in the P group. In MSC-2 and MSC-3 groups, submesothelial thickness was not different from C group.
Notes: *p < 0.01 PD versus C and p < 0.0001 P-2 and P-3 versus C; #p < 0.05 MSC-3 versus PD, p < 0.01 P-3 versus PD, and p < 0.0001 MSC-2 versus PD; +p < 0.0001 versus MSC-2; §p < 0.0001 versus MSC-3; xp < 0.05 MSC-3 versus P-2 and p < 0.01 P-3 versus P-2.
Effects of MSC Treatment on Cytokines and Growth Factors
Although a significant increase in both IL-6 and TNF-α concentrations in the MSC-2 group was observed compared to those of the others groups, MSC-3 group had lower levels of IL-6 and TNF-α compared with the others groups (p ≤ 0.001) except the P-3 group (p > 0.05). VEGF concentrations was lower in the MSC-3 and P-3 groups compared with MSC-2 (p < 0.0001) and PD (p = 0.04 and p = 0.03, respectively) groups. There was no statistically significant difference between groups in terms of TGF-β ().
Table 1. Cytokines and growth factors levels in the parietal peritoneum.
DISCUSSION
In this study, we evaluated the effects of MSC transplantation on inflammatory markers and morphological changes in a rat model of PD. To our knowledge, this is the first study regarding this topic. When MSC treatment was applied to rats undergoing PD, favorable changes were seen in morphology, but development of fibrosis was observed in the group without MSC treatment over time.
During the long-term PD, some structural and morphological changes occurred due to factors such as uremia, exposure to non-biocompatible dialysis solutions, or PD catheter.Citation1–3,5,10 As similar to our study, in a rat model for PD, it was seen that there was an increase in submesothelial thickness and number of inflammatory cells and vessels in the peritoneum after receiving PD fluid with 3.86% glucose by a dialysis catheter for 5 weeks.Citation11 Williams et al.Citation10 evaluated peritoneum of the patients on chronic PD and found increased submesothelial thickness, vasculopathy, reactivation in mesothelial cells, loss of mesothelial cells, and inflammation. Submesothelial thickness and grade of vasculopathy were increased, as the duration of PD was prolonged.Citation10 In our study, it was found that there were morphological changes, which were reflected by increased number of inflammatory cells, neovascularization, and thickened submesothelial due to chronic exposure of peritoneal membrane to standard PD fluid with high glucose concentration; thus, our findings support previous findings.
In chronic PD, non-biocompatible PD fluids and other various factors caused inflammation; thereby, mesothelial cells and macrophages were activated. This promotes production of growth factors and inflammatory cytokines. In turn, these factors activate fibroblasts, endothelial cells, and mast cells, which, in turn, release angiogenic and fibrotic cytokines and growth factors.Citation2,12 Maksic et al.Citation13 compared profiles of systemic and intraperitoneal pro-inflammatory cytokines and found that serum TNF-α levels were higher in patients receiving chronic PD than those with stages IV and V chronic renal failure without dialysis; and they also found that IL-6 level in PD fluid was higher in patients undergoing PD for longer than 1 year than those on PD for less than 1 year. Authors emphasized that duration of PD is of importance for increasing inflammation.Citation13 In our study, TNF-α and TGF-β levels were found to be higher in PD group than controls; additionally, an increase was seen in the number of inflammatory cells in PD group. Our findings indicated that chronic PD increase inflammation.Citation2,3,12,13
In the stem cell treatment, several labeling methods were used to determine whether tissue regeneration is driven by the effect of exogenous stem cells.Citation14–16 In our study, GFP labeling was employed and it was seen that GFP-labeled MSC was present in the mesothelium line at immunofluorescent microscopic evaluation. This finding supports the fact that changes seen in MSC group is caused by MSC given.
Inflammation plays a significant role in the injury of peritoneal membrane in chronic PD settings. Favorable effects of MSC usage were observed in many inflammation models.Citation8,9,17 In an experimental study, in which effects of MSC transplantation on inflammation were evaluated and cardiac function in an acute myocardial infarction model,Citation8 MSCs were promptly given via intracardiac route after inducing acute myocardial infarction. After 4 weeks, a decline was observed in the MSC group relative to myocardial infarction group in pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and a reduction was observed at infarct area; however, these cytokines were found to be equal or higher relative to control group.Citation8 In the bleomycin-induced acute lung injury model, it was seen that systemic administration of MSC caused significant reduction in inflammation, collagen deposit and matrix metalloproteinase activation, and prevented lung injury induced by bleomycin.Citation17 In this study, despite a borderline increase in inflammation was present in peritoneum of rats given MSC at week 2, morphological changes were not significantly different from control group at week 3. It may be considered that MSC had restorative/protective effect on peritoneum, since there was no increase in submesothelial thickness in both MSC groups and a marked increase in fibrosis in all groups without MSC treatment. The most intensive fibrosis was seen in P-3 group, followed by P-2 group. In PD group, submesothelial thickness was higher than control, MSC-2, and MSC-3 groups. Interestingly, marked increase was observed in pro-inflammatory cytokines at the week 2 after intraperitoneal MSC treatment. Elevation of pro-inflammatory cytokines at early period may be due to interactions occurred following MSC transplantation. Similar to our study, TNF-α and IL-6 levels were found to be higher in MSC group than controls at the week 2 after ischemia; however, on cerebral injury, restorative effects of MSC were observed during the same period.Citation18 Semedo et al.Citation19 injected MSC intravenously to the rats 6 hours after kidney injury induced by bilaterally clamping renal pedicles. They observed that expressions of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) were low 24 h after reperfusion, and expression of IL-6 was increased 48 h after reperfusion. They interpreted that this increase in IL-6 expression might be due to IL-6 expression of MSC itself.Citation19 Furthermore, Djouad et al.Citation20 observed that MSC secrete higher levels of IL-6 and VEGF when cultured in mixed lymphocyte reactions.
In our study, P group may be considered as peritoneal resting. While positive effects were observed on inflammation by peritoneal resting; especially, an increase in submesothelial thickness was prominent at the week 3. After peritoneal resting, observation of the deterioration may be related to ongoing injury for a time period, despite removal of the reason. In a study on rats, in which effects of peritoneal resting was assessed,Citation11 PD fluid with 3.86% glucose was administered daily for 5 weeks, followed by 12 weeks peritoneal resting period before assessments. The authors observed improvements in submesothelial thickness, cellular changes, and number of vessels.Citation11 In a study by Kim et al.,Citation21 PD fluid was given twice everyday during 3 weeks, followed by a 4-week peritoneal resting period. After peritoneal resting period, they found that submesothelial thickness was decreased; even it was higher than controls.Citation21 In our study, in which PD fluid administered for 6 weeks and assessment was made 2 and 3 weeks after resting period, the increase in submesothelial thickness was prominent at the third week of peritoneal resting period. Inconsistency of these findings with other investigations may be caused by longer treatment and shorter peritoneal resting periods in our study.
As a conclusion, we assessed the effects of MSC transplantation on pro-inflammatory cytokines, growth factors, and histological changes in the rat model of PD. It was seen that morphology of peritoneum was damaged in the group underwent PD and without MSC transplantation, whereas morphological changes other than inflammation in the MSC group were not different from control group. These findings suggest that MSC treatment may restore/prevent injury of peritoneal membrane. Moreover, one of the mechanisms underlying the therapeutic effect of MSC may be the regulation of inflammatory response in PD.
Our preliminary results reveal clues, implying that MSC transplantation is promising. Further experimental and clinical investigations are needed to assess the efficiency of MSC transplantation on preservation of peritoneal membrane or amelioration of peritoneal injury.
ACKNOWLEDGMENT
The authors thank Dr. Erman Dörterler for his technical assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported financially by the Erciyes University Research Fund.
REFERENCES
- Saxena R. Pathogenesis and treatment of peritoneal membrane failure. Pediatr Nephrol. 2008;23:695–703.
- Schilte MN, Celie JW, Wee PM, Beelen RH, van den Born J. Factors contributing to peritoneal tissue remodeling in peritoneal dialysis. Perit Dial Int. 2009;29:605–617.
- Fusshoeller A. Histomorphological and functional changes of the peritoneal membrane during long-term peritoneal dialysis. Pediatr Nephrol. 2008;23:19–25.
- Aroeira LS, Aguilera A, Sánchez-Tomero JA, . Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18:2004–2013.
- De Vriese AS, Mortier S, Lameire NH. What happens to the peritoneal membrane in long-term peritoneal dialysis? Perit Dial Int. 2001;21:9–18.
- Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123.
- Mimeault M, Batra SK. Concise review: recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–2345.
- Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104.
- Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863.
- Williams JD, Craig KJ, Topley N, . Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–479.
- Zareie M, Keuning ED, ter Wee PM, Beelen RH, van den Born J. Peritoneal dialysis fluid-induced changes of the peritoneal membrane are reversible after peritoneal rest in rats. Nephrol Dial Transplant. 2005;20:189–193.
- Lai KN, Leung JC. Inflammation in peritoneal dialysis. Nephron Clin Pract. 2010;116:c11–18.
- Maksic D, Vasilijic S, Colic M, Stankovic-Popovic V, Bokonjic D. Systemic and intraperitoneal proinflammatory cytokine profiles in patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial. 2009;25:50–55.
- Himmelreich U, Dresselaers T. Cell labeling and tracking for experimental models using magnetic resonance imaging. Methods. 2009;48:112–124.
- Arbab AS, Janic B, Haller J, Pawelczyk E, Liu W, Frank JA. In vivo cellular imaging for translational medical research. Curr Med Imaging Rev. 2009;5:19–38.
- Izuta Y, Ochi M, Adachi N, Deie M, Yamasaki T, Shinomiya R. Meniscal repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee. 2005;12:217–223.
- Ortiz LA, Gambelli F, McBride C, . Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411.
- Gutiérrez-Fernández M, Rodríguez-Frutos B, Alvarez-Grech J, . Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405.
- Semedo P, Palasio CG, Oliveira CD, . Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009;9:677–682.
- Djouad F, Charbonnier LM, Bouffi C, . Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032.
- Kim YL, Kim SH, Kim JH, . Effects of peritoneal rest on peritoneal transport and peritoneal membrane thickening in continuous ambulatory peritoneal dialysis rats. Perit Dial Int. 1999;19:384–387.