Abstract
Background: Urinary calculi are a common and severe problem, which are formed by urolithiasis or by the formation of calcium oxalate (CaOx) crystals in the kidneys. Many treatment options such as drugs, various herbal preparations, surgical removal of the stones, and extracorporeal shock wave lithotripsy have been applied for this condition. The aim of this study is to assess the effects of the drug amlodipine in an experimentally induced urolithiasis rat model. Materials and methods: The effect of 5 mg/kg amlodipine was studied in rats that were first treated with 1% ethylene glycol and 1% ammonium chloride for 21 days to induce urolithiasis. The weight differences and the levels of calcium, magnesium, and phosphate were measured in serum and urine. In addition, urine CaOx level was defined and histopathological analyses were performed on the kidneys. Results: Urolithiasis caused a significant increase in both serum and urine parameters compared with healthy rats. Urolithiasis plus amlodipine administration increased the levels of these same parameters. Urine CaOx level was high in urolithiasis rats and was also increased by urolithiasis plus amlodipine administration. The weight of the rats decreased in the urolithiasis plus amlodipine group when compared with the urolithiasis group. Histopathological examinations revealed extensive intratubular crystal depositions and degenerative tubular structures in the urolithiasis group and the amlodipine treatment group. Conclusion: We showed that amlodipine may increase susceptibility to urolithiasis by raising hyperoxaluria and hypercalciuria. Further studies should be performed to elucidate the urolithiasis activity of amlodipine and to confirm the data.
INTRODUCTION
Urolithiasis, also known as calculi or uroliths, is a condition that involves stone formation in the kidneys, bladder, and/or urethra. It is one of the major causes of labor loss and is endemic worldwide. It is increasing especially in the tropics similarly to most Western countries with a reported incidence of approximately 12% in the general population.Citation1 Various in vivo methods and therapeutic agents were used to provide the development and progression of the urolithiasis disease.Citation2 The most common model is ethylene glycol (EG)- and ammonium chloride (AC)-induced urolithiasis model. This model is a very economic, simply applicable (used as rat drinking water) and reliable method.Citation3 EG is commonly used in automotive products, such as brake fluids, anti-freeze, windshield de-icers, and coolants.Citation4–6 Because EG has a sweet taste,Citation7 it can contribute to substance addiction, suicide attempts, and the accidental poisoning of children.Citation8 EG is not actually toxic, although its toxicity originates from toxic metabolites.Citation5 EG is oxidized to glycolic acid which, in turn, is oxidized to the toxic compound oxalic acid (oxalate).Citation9 Glycolic acid causes severe acidosis, and oxalate precipitates as calcium oxalate (CaOx) in the kidneys that leads to the storage of CaOx crystals and the formation of kidney stones.Citation5,6,10
Amlodipine is one of the most common of calcium antagonists and is used in hypertensive populations all over the world. Amlodipine is a dihydropyridine calcium antagonist that inhibits the transmembrane influx of calcium ions into vascular smooth muscle.Citation11 It is used clinically as a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure. One of the effects of dihydropyridine group drugs is their weak diuretic effects.Citation12
It was reported that calcium antagonists had a protective effect against kidney stone production and a curative effect on existing kidney stones.Citation13 In addition, it was stated that amlodipine has an oxalate store-depleted effect in the kidneys of normotensive hyperoxaluric rats.Citation14
Therefore, in this study, it was aimed to study the biochemical and histopathological effects of amlodipine in EG-induced urolithiasis model in rat urine, serum, and kidney tissue.
MATERIALS AND METHODS
Chemicals
Amlodipine (Norvasc, 5 mg tablet) was purchased from Pfizer (Istanbul, Turkey) and thiopental sodium (Pentothal sodium, 1 g) was purchased from Abbott, in Istanbul, Turkey. All of the other chemicals were obtained from Sigma Co. (Cream Ridge, NJ, USA).
Experimental Animals
A total of 24 male Sprague-Dawley rats, weighing 180–200 g, were used in the experiments. The experiments were conducted according to the ethical norms approved by the Ethic Committee of Experimental Animal Teaching and Researcher Centre (No: B.30.2.ATA.0.23.85-71). Rats were obtained from the Medical and Experimental Application and Research Centre (ATADEM), Erzurum, Turkey. They were kept in standard laboratory conditions under natural light and dark cycles. Animals were fed a normal diet and water ad libitum before the experiments.
Experimental Protocols for EG- and AC-Induced Urolithiasis in Rats
Rats were divided into four groups (6 rats per group) and all the groups were maintained using commercial pelleted feed. To induce CaOx crystal formation, animals were exposed to 1% EG and 1% AC in their drinking water for 21 days. The experimental groups are summarized in .
Table 1. Experimental protocol for urolithiasis and treatment groups.
In the intact control group (Group 1), animals were given tap water as their drinking water for 21 days. The urolithiasis group (Group 2) animals were exposed to 1% EG with 1% AC in their drinking water for 21 days. In the amlodipine (5 mg/kg) group (Group 2), rats were orally administered amlodipine (5 mg/kg) daily for 21 days. This group also received tap water during experiment. In the amlodipine treatment group (Group 4), animals were exposed to 1% EG and 1% AC in their drinking water and 5 mg/kg dose of amlodipine dissolved with saline was administered daily by gavage for 21 days (). All the rats were weighed weekly.
Twenty-four-hour urine samples were collected with sodium azide as a preservative from rats housed in metabolic cages on the 21st day of the experiment. Blood was collected in tubes without anticoagulant for serum samples. Serum was separated by centrifugation. At the end of the experimental period, the animals were slaughtered using high-dose sodium thiopental (50 mg/kg) and their abdomens were opened and the kidneys were removed. The kidneys were fixed in 10% formalin.
Biochemical Assays in Urine and Serum
The separated serum and urine samples were used to determinate calcium, potassium, and magnesium levels using an auto-analyzer (Comas 6000; Roche, Tokyo, Japan). The measurements were in mg/dL for all three minerals.
Microscopy and Chemistry of Urine
The levels of CaOx and pH in the urine samples were determined using an IQ-200 Automated Urine Microscopy Analyzer and an Aution Max AX-4280 Automated Urine Chemistry Analyzer. For the urine sediment analysis, 10 high-power fields (HPF) were estimated and the counts were given as an average per HPF. These values were normalized to a given volume.
Histological Procedures for Light Microscopy
Each kidney was fixed in 10% formalin solution for 48–55 hours, dehydrated in a graded alcohol series, embedded in paraffin wax, and serially sectioned using a microtome (Leica RM2125RT). The sections were stained using H&E (Hematoxylin and Eosin) staining and photographs were taken using a light microscope with a camera attachment (Nikon Eclipse E600, Tokyo, Japan).
Statistical Analysis
Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by LSD test using IBM SPSS statistics software package, version 19.00. p-Values of <0.05 were considered as significant. The results are expressed as mean ± standard deviation (SD) for 6 rats in each group.
RESULTS
Serum and Urine Analysis
The effects of amlodipine on serum and urine levels of calcium, potassium, and magnesium are shown in and , respectively. All parameters were found to be statistically (p < 0.05) different in all groups when compared with the urolithiasis group. The urolithiasis plus amlodipine group increased the levels of all parameters with the exception of the potassium level () when compared with both the intact and the urolithiasis control groups. The urolithiasis plus amlodipine group showed a decreased serum potassium level. However, the calcium, potassium, and magnesium levels in the serum were not significantly affected when amlodipine alone (intact amlodipine group, 5 mg/kg body weight) was administrated to the intact group ().
Table 2. The levels of Ca, P, and Mg in the serum of experimental groups.
Table 3. The levels of Ca, P, and Mg in the urine of experimental groups.
Calcium Oxalate in Urine
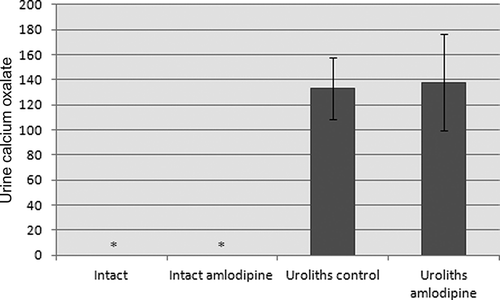
Automated Urine Microscopy Analyzer determination revealed that after the 21-day experimental period, there was no crystal formation in urine from the intact control and the amlodipine alone groups. Whereas, the urine sample revealed the presence of abundant CaOx crystals and the aggregates were quite visible in many instances in the urolithiasis plus amlodipine and the urolithiasis groups (). Urine CaOx levels in the urolithiasis plus amlodipine and the urolithiasis groups increased significantly (p < 0.001) when compared with the intact groups ().
Figure 1. Urine CaOx levels in urolithiasis rats treated with amlodipine.
Notes: Each value is mean ± SD for 6 rats in each group. Statistical analysis was carried out using one-way ANOVA followed by LSD test. Intact control, intact amlodipine, and amlodipine plus urolithiasis groups were compared with the urolithiasis group.*Significant at p < 0.05.
Body Weight
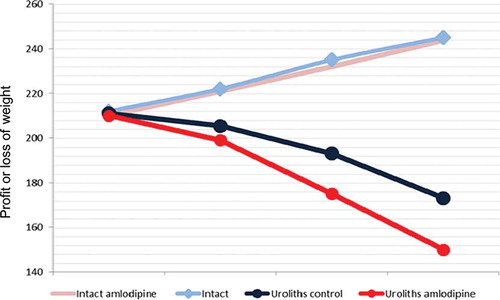
Percentage changes in body weight in all rat groups over the 21 days are presented in . The rats in the urolithiasis plus amlodipine and the urolithiasis groups lost their body weight significantly for 3 weeks. Also, body weight levels in the urolithiasis plus amlodipine group decreased significantly (p < 0.05) when compared with the urolithiasis group (). However, all of the rats in the intact groups had increased body weights.
Figure 2. Percentage changes in body weight of the rat groups over 21 days of the experiment.
Notes: Each value is mean for 6 rats in each group. Statistical analysis for the third weekend weight was carried out using one-way ANOVA followed by LSD. Intact control, intact amlodipine, and urolithiasis plus amlodipine groups were compared with the urolithiasis group.*Significant at p < 0.05.
Histopathological Results
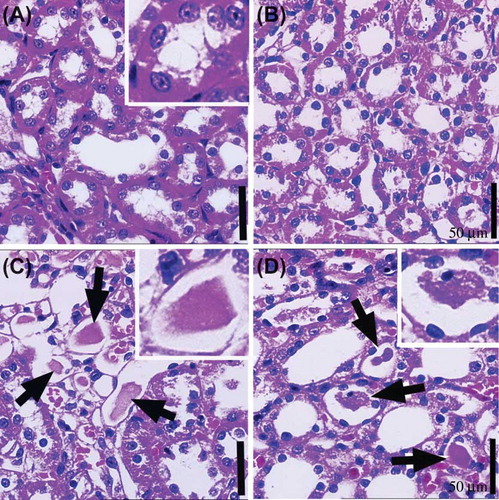
The general structure of the kidney in the intact control and the intact amlodipine (5 mg/kg) treated rats were normal (A and B). Extensive intratubular crystal depositions and degenerative tubular structures were found in urolithiasis and amlodipine plus urolithiasis rats (C and D). There were no intratubular crystal deposits or histopathological changes in both intact groups (intact control and intact amlodipine) (A and B). However, there were some cells with heterochromatic nucleus (dark staining nucleus) in the tubular walls in the urolithiasis plus amlodipine group (D).
Figure 3. The medullary area of kidney tissue in (A) the intact control group, (B) intact amlodipine group, (C) urolithiasis group, and (D) amlodipine plus urolithiasis group.
Note: Crystal deposits (white arrow and square area) are observed in the intratubular area and degenerative tubular structures in the medullary area of the (C) urolithiasis model group and (D) amlodipine plus urolithiasis group.Scale bar: 50 μm.
DISCUSSION
The majority of kidney stones are made up of CaOx crystals in the urinary system of patients with urolithiasis. In countries where there is also a high intake of oxalates from local leaves and vegetables, urinary oxalate is increased and, as a result, the ammonium acid urine stones often contain CaOx as well. As living standards increase, particularly in the urban areas of the more affluent developing countries, so too do the incidence of upper urinary tract stones being increasingly characterized by CaOx stones that are often mixed with calcium phosphate and uric acid. Obligado and Goldfarb reported that patients with hypertension may have abnormalities in renal calcium metabolism, although the authors also reported that the data to confirm this hypothesis was inconsistent.Citation15 Domingos and Serra reported that nephrolithiasis was associated with an increased prevalence of cardiovascular disease, especially with hypertension, obesity, and diabetes mellitus.Citation10
Hypertension prevalence is approximately 15–20% in the general population and approximately 26% in the adult population.Citation16 The prevalence of hypertension increases with advancing age. For example, approximately 50% of people between the ages of 60 and 69 years have hypertension, and the prevalence is further increased with age.Citation17 Various effective treatments for hypertension are available. The basic treatment goals include reducing elevated blood pressure, improving blood pressure control, treating resistant hypertension, reducing associated cardiovascular risk factors, and reducing renal damage. Many agents are in use for hypertension treatment. Among these, amlodipine is one of the most popular agents for hypertension patients and is in use worldwide.
Few studies are present concerning the effect of calcium agonists in EG-induced urolithiasis models. In clinical and experimental studies, it was propounded that calcium antagonists had a partial protective and curative effect on kidney stones.Citation13,14,18,19 However, it was suggested in some studies that amlodipine caused hyperoxaluria.Citation18 One experimental study stated that amlodipine decreased hyperoxaluria and had a protective effect in the kidney tubular system.Citation4 However, we experimentally determined that amlodipine showed a contrary effect to the previous study in an EG- and AC-induced CaOx stone model. This result is in accordance with the histopathological and biochemical results (). In other words, an increase in hyperoxaluria was shown with the use of amlodipine in our study.
It is clear that stone disease risk increases with dehydration.Citation20 Hence, physicians suggest their patients to take much water to increase their urine output.Citation21 In our study, we have found that amlodipine group loses much more weight than the other groups. As a result of our experimental method, both EG and AC might have caused a strong dehydration on rats. In urolithiasis plus amlodipine group, increased dehydration might have caused by synergic effect of EG/AC and the diuretic effect of amlodipine, in this experimental study.Citation12,22 Considering its diuretic effect, the amlodipine might have promoted the stone accumulation.
It was shown that severe dehydration and weight decrease (mean %) were also observed in the urolithiasis plus amlodipine group (). Because there are few studies on this topic, we think that it is still controversial. There can be several explanations for the results. First, calcium antagonists may not be useful in the treatment of kidney stones. Second, the weight loss observed may originate from the diuretic effects of the drugs.Citation12,22 Because EG exposure is common in society, clinicians should be prudent when suggesting calcium antagonists to treat hypertension patients with EG exposure because of the increased risk of contracting kidney stones. Furthermore, we showed that Ca, Mg, P, and oxalate levels were high in serum and in the urine of the rat model that were administered amlodipine ( and , ). It is well known that increase in the urine calcium level causes the stone accumulation.Citation23 Also, in case of hyperoxaluria, propensity to urolithiasis is promoted.Citation24 It is recently reported that an increase in the urine magnesium output level may decrease the CaOx crystal formation,Citation25 while an increase in urine phosphate output level increases Ca and P stones.Citation23 It seems to have more complicated mechanisms causing this result. For example, one approach might be an increase in the serum and urine Ca levels, promoting the stone formation, as a result of transmembrane influx of calcium ions blockage caused by amlodipine. Although opposite to consideration, we observed that amlodipine may be useless because EG converts to glyoxylic acid and binds to increased extracellular calcium, which that may contributes to CaOx formation.
CONCLUSION
It was shown that amlodipine may increase susceptibility to urolithiasis by increasing hyperoxaluria and hypercalciuria. Therefore, this study has shown experimentally that amlodipine has no positive effects on EG-induced stone formation in rats, contrary to previous studies. Further clinical and experimental studies should be performed to clarify this issue.
ACKNOWLEDGMENT
This research was conducted in the Research Laboratory of Basic and Clinical Medical Sciences and Drug Development at Ataturk University, School of Medicine, Department of Pharmacology, 25240, Erzurum, Turkey.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was supported by Ataturk University Scientific Research Council (BAP) (grant number BAP: 2011/293).
REFERENCES
- Araujo Viel T, Diogo Domingos C, da Silva Monteiro AP, Riggio Lima-Landman MT, Lapa AJ, Souccar C. Evaluation of the antiurolithiatic activity of the extract of Costus spiralis Roscoe in rats. J Ethnopharmacol. 1999;66:193–198.
- Touhami M, Laroubi A, Elhabazi K., Lemon juice has protective activity in a rat urolithiasis model. BMC Urology. 2007;7:18.
- Bayir Y, Halici Z, Keles MS., Helichrysum plicatum DC. subsp. plicatum extract as a preventive agent in experimentally induced urolithiasis model. J Ethnopharmacol. 2011;138:408–414.
- Montjoy CA, Rahman A, Teba L. Ethylene glycol and methanol poisonings: case series and review. W V Med J. 2010;106:17–23.
- Lovric M, Granic P, Cubrilo-Turek M, Lalic Z, Sertic J. Ethylene glycol poisoning. Forensic Sci Int. 2007;170:213–215.
- Wollersen H, Erdmann F, Risse M, Dettmeyer R. Oxalate-crystals in different tissues following intoxication with ethylene glycol: three case reports. Leg Med (Tokyo). 2009;11(Suppl. 1):S488–S490.
- Curtin L, Kraner J, Wine H, Savitt D, Abuelo JG. Complete recovery after massive ethylene glycol ingestion. Arch Intern Med. 1992;152:1311–1313.
- White NC, Litovitz T, Benson BE, Horowitz BZ, Marr-Lyon L, White MK. The impact of bittering agents on pediatric ingestions of antifreeze. Clin Pediatr. 2009;48:913–921.
- Hatchett R. A severe and fatal case of ethylene glycol poisoning. Intensive Crit Care Nurs. 1993;9:183–190.
- Domingos F, Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant. 2011;26:864–868.
- Sahney S. A review of calcium channel antagonists in the treatment of pediatric hypertension. Paediatr Drugs. 2006;8:357–373.
- Carter AJ, Gardiner DG, Burges RA. Natriuretic activity of amlodipine, diltiazem, and nitrendipine in saline-loaded anesthetized dogs. J Cardiovasc Pharmacol. 1988;12(Suppl. 7):S34–S38.
- Sarica K, Inal Y, Erturhan S, Yagci F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urol Res. 2006;34:184–189.
- Toblli JE, Ferder L, Angerosa M, Inserra F. Effects of amlodipine on tubulointerstitial lesions in normotensive hyperoxaluric rats. Hypertension. 1999;34:854–858.
- Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. Am J Hypertens. 2008;21:257–264.
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223.
- Chobanian AV, Bakris GL, Black HR., Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252.
- Sarica K, Erturhan S, Altay B. Effect of verapamil on urinary stone-forming risk factors. Urol Res. 2007;35:23–27.
- Beach MA, Mauro LS. Pharmacologic expulsive treatment of ureteral calculi. Ann Pharmacother. 2006;40:1361–1368.
- Borissova A, Goltz GE, Kavanagh JP, Wilkins TA. Reverse engineering the kidney: modelling calcium oxalate monohydrate crystallization in the nephron. Med Biol Eng Comput. 2010;48:649–659.
- Hussain M, Rizvi SA, Askari H., Management of stone disease: 17 years experience of a stone clinic in a developing country. J Pak Med Assoc. 2009;59:843–846.
- Johns EJ. A study of the renal actions of amlodipine in the normotensive and spontaneously hypertensive rat. Br J Pharmacol. 1988;94:311–318.
- Sorensen MD, Duh QY, Grogan RH, Tran TC, Stoller ML. Urinary parameters as predictors of primary hyperparathyroidism in patients with nephrolithiasis. J Urol. 2012;187:516–521.
- Khan SR, Glenton PA. Experimental induction of calcium oxalate nephrolithiasis in mice. J Urol. 2010;184:1189–1196.
- Spasov AA, Iezhitsa IN, Kharitonova MV, Kravchenko MS. Effects of magnesium salts on the course of experimental calcium-oxalate urolithiasis. Urologiia. 2011;2:23–29.
