Abstract
Arsenic is a terribly poisonous material. There have been many reports of arsine poisoning in workers, and a few have discussed acute kidney injury by arsine. But literatures which investigated the pathologic findings are uncommon, and especially, the ones describing ultrastructural findings are rare. Here, we report an incident of acute arsine poisoning complicated by acute kidney injury and suggest the characteristics of the renal pathology in arsine-induced renal injury, especially the ultrastructural findings.
INTRODUCTION
Arsenic is a toxic material that can cause various organ dysfunctions. In the modern industrial process, particularly, workers are occasionally poisoned by arsine from the process.Citation1 There have been many reports of arsine poisoning in workers, and a few have discussed acute kidney injury by arsine. But literatures which investigated the pathologic findings are uncommon,Citation2–4 and the ones describing ultrastructural findings are especially rare.Citation5,6 Here, we report on an incident of acute arsine poisoning complicated by acute kidney injury and suggest the characteristics of the renal pathology in arsine-induced renal injury, especially the ultrastructural findings.
CASE REPORT
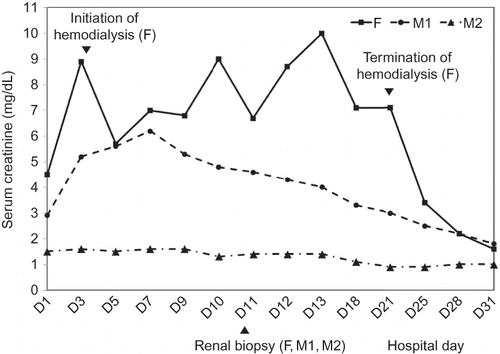
Three laborers at a metal smelting factory (one 45-year-old female and two 63-year-old males) were admitted complaining of nausea, vomiting, and gross hematuria that had started 2 days prior to their visit. Before the symptoms had started, the patients had, over a 2-h period, used sulfuric acid to remove the metal residue from the smelting process. Initial blood tests showed hemoglobin levels to be 10.5, 8.7, and 9.5 g/dL (reference value: female, 12.0–16.0 g/dL; male, 13.5–17.5 g/dL) (further results are listed in the following order: female, first male, and second male). And haptoglobin levels were reduced to 7, 1, and 3 mg/dL (reference value, 16–199 mg/dL), whereas plasma hemoglobin levels were increased to 217, 27, and 132 mg/dL (reference value, 1–5 mg/dL). These findings suggested hemolytic anemia. In the serum biochemistry test, all of the patients’ electrolytes were found to be within the normal range. But urea nitrogen levels were elevated to 83, 67, and 64 mg/dL (reference value, 10–20 mg/dL) and creatinine levels were increased to 4.5, 2.9, and 1.5 mg/dL (reference value, <1.5 mg/dL), indicating acute kidney injury. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were elevated to 397 and 8500 and 144, and 141 and 4 U/L (reference value: both AST and ALT, 0–35 U/L), and creatinine kinase (CK) levels were elevated to 786, 469, and 416 U/L (reference value: female, 40–150 U/L; male, 60–400 U/L), suggesting rhabdomyolysis. In the urine analysis, findings of hematuria, proteinuria, and low-level glucosuria were shown in all patients. In addition, urine β2-microglobulins in all patients were elevated to >4.0 mg/L (reference value, 0–0.15 mg/L), suggesting renal tubular injury. In the 24-h urine test, proteinuria levels were measured to be 1286, 824, and 1062 mg/day, which showed a tubular proteinuria in the electrophoresis test. In blood tests to confirm heavy metal poisoning, cadmium (Cd) levels were 3.6, 2.8, and 2.0 μg/L (reference value: cadmium handler, <5.0 μg/L). In urine tests, arsenic (As) levels in the single voided urine specimen were 467.58, 624.55, and 546.07 μg/L (reference value, 0–52.7 μg/L). Arsenic values in the 24-h urine specimens were 141.1, 2069.9, and 1689.0 μg/day (reference value, <120 μg/day) and cadmium values were shown to be 6.0, 148.7, and 202.4 μg/day (reference value, <3.0 μg/day), which suggested acute arsenic poisoning and chronic cadmium exposure. All patients were hospitalized. The male patients had no symptoms relating to renal failure, but the female patient experienced oliguria. Her serum urea nitrogen and creatinine levels were found to be elevated to 141 and 8.9 mg/dL, and pulmonary edema was developed. Therefore, hemodialysis was initiated. On the tenth day of admission, kidney biopsies were performed on all patients to investigate the pathologic findings of renal injury. After 2 weeks of hemodialysis, the urine output of the female patient had increased, and thus hemodialysis was terminated. Renal functions in all patients gradually improved, and they were discharged (). All patients were not initially treated with chelating agents because we had difficulty in getting medicine. However, we could obtain the chelating agent (succimer) after patient’s discharge and gave it to them for persistent elevation of arsenic concentrations in urine. Three months after the initial treatment, the creatinine levels in the three patients have been normalized without the complication of chronic kidney disease and the concentrations of arsenic in urine have returned to normal. Therefore, the follow-up was terminated.
DISCUSSION
We described the case of three workers who were poisoned by arsine. All were engaged in a metal residue cleaning process using sulfuric acid, and it appeared that they were poisoned by arsine generated during the cleaning process. All patients showed hemolytic anemia, rhabdomyolysis, and acute kidney injury by arsine poisoning. In the heavy metal test, the amount of cadmium, as well as arsenic, was shown to have significantly increased in the 24-h urine specimen. Nevertheless, we concluded that the acute symptoms of the patients were not caused by cadmium but by arsine, because the acute symptoms observed in our case were more compatible with those seen in arsine poisoning. In particular, hemolytic anemia is specific to arsine poisoning. Therefore, we concluded that the increased cadmium in the urine was due to chronic exposure.
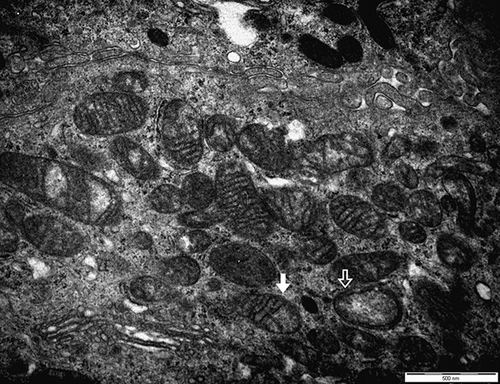
In the kidney biopsies, acute interstitial nephritis was confirmed. Similarly to previous reports, our case also showed that renal tubular cells were damaged and interstitium was infiltrated by inflammatory cells. Glomeruli appeared normal ().Citation2–6 Under electron microscopy, a few focal fusions of podocyte processes in the glomerulus were seen, but they were not significant and considered nearly normal. In the tubular cells, the mitochondria became diverse in shape, smaller in size, and had a dense matrix, which suggested degeneration (). In addition, a few mitochondria showed that the outer membranes had ruptured and their cristae were disrupted (). We considered these features of mitochondria were important in arsine-induced renal pathology, because according to previous reports, the mitochondria has been known to be a specific target in arsenic-induced injury, although it is not exclusive.Citation7,8 In addition, other ultrastructural findings included many vacuolar structures containing dense granular materials (). We considered it to be the lysosomes swallowing the toxic arsenic materials.Citation5,6 According to Fowler et al., in renal tubular damage by arsenic, lysosomes were suggested to be the place in which arsenic–hemoglobin complexes were sequestered for long periods.Citation9 Therefore, we also considered that dense granules in the vacuolar structures might be the arsenic–hemoglobin complexes.
Figure 2. Light microscopy shows that the glomerulus appears normal and tubular epithelial cells are degenerated with focal loss of nuclei. Some tubular lumens are dilated and filled with eosinophilic casts. Interstitia are slightly widened with a few mononuclear cells (H&E, ×200).
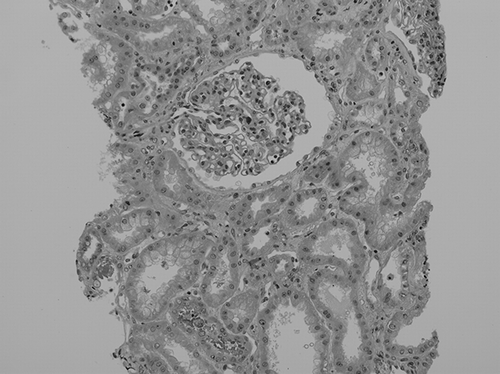
Figure 3. The section shows many lysosomes containing dense granular materials (black arrow). Equal-sized oval mitochondria are considered to be normal (white arrow). Diverse-shaped and small-sized mitochondria that have a dense matrix are considered to be degenerated (hollow arrow) (second male, electron microscopy, ×10,000).
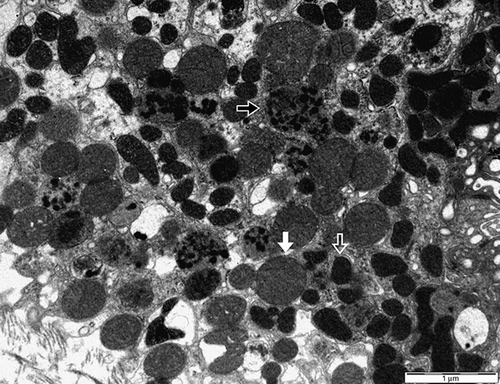
Figure 4. The section shows that the outer membranes of mitochondria are ruptured and the cristae are disrupted (white arrow). A few mitochondria show vacuolated changes (hollow arrow) (first male, electron microscopy, ×20,000).
In conclusion, we reported on the cases of acute arsine poisoning complicated by acute kidney injury and suggested the characteristics of the renal pathology in arsine-induced renal injury, especially the ultrastructural pathologic findings.
ACKNOWLEDGMENTS
We thank Dr. Tae-jung Kwon of the National Forensic Service for his advice on electronic microscopic findings.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- Pullen-James S, Woods SE. Occupational arsine gas exposure. J Natl Med Assoc. 2006;98(12):1998–2001.
- Uldall PR, Khan HA, Ennis JE, McCallum RI, Grimson TA. Renal damage from industrial arisine poisoning. Br J Ind Med. 1970;27(4):372–377.
- Gerhardt RE, Hudson JB, Rao RN, Sobel RE. Chronic renal insufficiency from cortical necrosis induced by arsenic poisoning. Arch Intern Med. 1978;138(8):1267–1269.
- Prasad GV, Rossi NF. Arsenic intoxication associated with tubulointerstitial nephritis. Am J Kidney Dis. 1995;26(2):373–376.
- Muehrcke RC, Pirani CL. Arsine-induced anuria. A correlative clinicopathological study with electron microscopic observations. Ann Intern Med. 1968;68(4):853–866.
- Zhang L, Zhang Y, Lin Q, . Quiz page December 2008. Acute kidney injury, hematuria, and orange skin pigmentation caused by arsine poisoning. Am J Kidney Dis. 2008;52(6):A33–A36.
- Bustamante J, Nutt L, Orrenius S, Gogvadze V. Arsenic stimulates release of cytochrome c from isolated mitochondria via induction of mitochondrial permeability transition. Toxicol Appl Pharmacol. 2005;207(Suppl. 2):110–116.
- Wang Y, Xu Y, Wang H, . Arsenic induces mitochondria-dependent apoptosis by reactive oxygen species generation rather than glutathione depletion in Chang human hepatocytes. Arch Toxicol. 2009;83(10):899–908.
- Fowler BA, Weissberg JB. Arsine poisoning. N Engl J Med. 1974;291(22):1171–1174.