Abstract
All-trans retinoic acid (ATRA) is an important therapeutic agent for prevention of the renal diseases. Transforming growth factor-β1 (TGF-β1)/Smad3 signaling pathway is a key signaling pathway which takes part in the progression of renal interstitial fibrosis (RIF). This investigation was performed to study the effect of ATRA in RIF rats and its effect on the TGF-β1/Smad3 signaling pathway. Sixty Wistar male rats were divided into three groups at random: sham operation group (SHO), model group subjected to unilateral ureteral obstruction (GU), model group treated with ATRA (GA), n = 20, respectively. RIF index, protein expression of TGF-β1, collagen-IV (Col-IV) and fibronectin (FN) in renal interstitium, and mRNA and protein expressions of Smad3 in renal tissue were detected at 14-day and 28-day after surgery. The RIF index was markedly elevated in group GU than in SHO group (p < 0.01), and the RIF index of GA group was alleviated when compared with that in GU group (p < 0.01). Compared with in group SHO, the mRNA/protein expression of Smad3 in renal tissue was significantly increased in group GU (p < 0.01). However, the mRNA and protein expressions of Smad3 in renal tissue in GA group were not markedly alleviated by ATRA treatment when compared with those in GU (each p > 0.05). Protein expressions of TGF-β1, Col-IV, and FN in GU group were markedly increased than those in SHO group (each p < 0.01), and their expressions in GA group were markedly down-regulated by ATRA treatment than those of GU group (all p < 0.01). The protein expression of Smad3 was positively correlated with RIF index, protein expression of TGF-β1, Col-IV or FN (each p < 0.01). In conclusion, ATRA treatment can alleviate the RIF progression in UUO rats. However, ATRA cannot affect the signaling pathway of TGF-β1/Smad3 in the progression of RIF.
INTRODUCTION
All-trans retinoic acid (ATRA) is a biologically active metabolite of vitamin A. ATRA serves as a critical signaling molecule, regulating gene transcription, cell division, and apoptosis during development and adult life.Citation1 Multiple functions of ATRA in various physiological systems have engendered interest beyond the traditional retinoid attention areas of cancer and development.Citation2 Interest in ATRA action has increased considerably concerning the nervous system, the immune system, the uropoietic system, energy balance, and obesity.Citation2,3 Some investigations found that ATRA had a preventive effect on renal fibrosis,Citation4 pulmonary fibrosis,Citation5 liver fibrosis,Citation6 and so on.
Smad3 is an intracellular signaling molecule in the transforming growth factor β (TGF-β) pathwayCitation7 and is considered as a downstream mediator of TGF-β signaling.Citation8 TGF-β/Smad3 signaling plays a central role in tissue fibrogenesis, acting as a potent stimulus of extracellular matrix accumulation.Citation9 Transforming growth factor-β1 (TGF-β1) is an important subtype of TGF-β. Increasing evidence shows that TGF-β1 is a key mediator in the pathogenesis of renal fibrosis in both experimental and human kidney diseases because TGF-β1 is highly up-regulated in the diseased kidney with severe renal fibrosis.Citation10 As those mentioned above, TGF-β/Smad3 signaling pathway might take part in the pathogenesis of renal fibrosis.
Whether the ATRA could play a protective role by TGF-β/Smad3 signaling pathway in the pathogenesis of renal fibrosis, this study was performed to explore this relationship in renal interstitial fibrosis (RIF) rats induced by unilateral ureteral obstruction (UUO).
MATERIALS AND METHODS
Animal Model
Sixty male Wistar rats (6-week-old) were obtained from the Experimental Animal center of Guangxi Medical University, Nanning, China. The rats were divided into three groups at random: sham operation group (SHO), model group subjected to unilateral ureteral obstruction (GU), model group treated with ATRA (GA), n = 20, respectively. The ureter was ligated at approximately 1 cm below the renal hilum with 3–0 silk suture. The abdominal wound was closed, and rats were returned to the cages. Control rats underwent abdominal incision and approximation with no ligation of the ureter.Citation11 Ten rats of the three groups were killed on 14-day and 28-day after surgery, respectively and their renal tissues were collected for histological and molecular biology determination.
All the protocols in this investigation were approved by the Animal Care and Use Committee of Guangxi Medical University.
Renal Morphology
The renal tissues were fixed using 10% neutral formaldehyde and were dehydrated through a graded ethanol series and embedded in paraffin. All the sections were prepared on a microtome and were stained with Masson’s trichrome staining. Renal pathology was observed by light microscope, the severity of the renal lesion was presented by the RIF index. Blue granular or linear deposits were interpreted as positive areas for collagen staining. Semi-quantitative evaluation was performed by a computer-aided manipulator (DMR + Q550, Leica Co., Wetzlar, Germany). The area of positive staining for fibrosis was measured at 400-folds original magnification in five fields (ignored the fields containing glomerular parts) and expressed as a percentage of the total area.Citation12 The extent of interstitial fibrosis was scored as absent (0), involving less than 25% of the area (1), involving 26–50% of the area (2), and involving greater than 50% of the area (3).Citation13 RIF index was obtained by the formula as follow: RIF = (0 × n0 + 1 × n1 + 2 × n2 + 3 × n3)/ (n0 + n1 + n2 + n3) = (0 × n0 + 1 × n1 + 2 × n2 + 3 × n3)/50. All the fields were selected from coded sections for each rat at random and the scores obtained by two investigators were averaged.
Immunohistochemical Analysis of the Protein Expressions of TGF-β1, Collagen-IV (Col-IV) and Fibronectin (FN)
Renal tissue fixed with 4% buffered paraformaldehyde was embedded in paraffin, and 4-μm thick sections were stained. The positive area was measured quantitatively using a computer-aided manipulator (DMR + Q550, Leica Co.). For immunohistochemical analysis of TGF-β1, Col- IV, and FN, the sections were deparaffinized, washed with PBS, and treated with 3% H2O2 in methanol for 10 min. All sections were then incubated with anti-TGF-β1 antibody (1:100) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-Col-IV antibody (1:100) (Bo Ao sen, Co., Bejing, China) and anti-FN antibody (1:100) (Bo Ao sen, Co., Bejing, China), respectively. All the sections were incubated with rabbit anti-mouse biotinylated second antibody immunoglobulin (IgG) for 30 min, followed by the avidin–biotin peroxidase complex (Maixin Bio, Co., Fuzhou, China). After the incubation, the sections were stained with diaminobenzidine (Maixin Bio, Co.). The positive area of TGF-β1, Col-IV or FN in renal tissue was measured. During evaluation of the interstitial areas, fields containing glomerular parts were ignored. All the evaluations were performed by two of the authors blinded to the experimental code.
Real Time Reverse Transcription Polymerase Chain Reaction to Detect PHB mRNA Expression in Renal Tissue
Renal tissue was homogenized and total RNA was extracted with TRIzol (Beijing Tiangen, Co., Beijing,China). Ultraviolet spectrophotometer measuring absorbance, agarose gel electrophoresis confirmed that there had been no degradation of RNA by visualizing the 18S and 28S RNA bands under ultraviolet light.Citation14,15 Primers were designed according to primer design principles by Primer Premier 5.0. The primers for Smad3 and internal control β-actin were as follows: F 5′-CTGGCTACCTGAGTGAAGATG-3′ and R 5′-TGTGAAGCGTGGAATGTCTC-3′ for Smad3; F 5′-GCCCCTGAGGAGCACCCTGT-3′ and R 5′-ACGCTCGGTCAGGATCTTCA-3′ for β-actin. One microgram total RNA from the renal tissue of each rat was reverse transcribed into cDNA with an ExScript RT reagent kit (Fermentas, Co., Vilnius, Lithuania). Smad3 and β-actin were amplified with SYBR Premix Ex Taq (Beijing Tiangen, Co.). Gene expression of β-actin was also measured in each sample and used as an internal control for loading and reverse transcription efficiency. The analysis for each sample was performed in triplicate. The average threshold cycle (Ct, the cycles of template amplification to the threshold) was worked out as the value of each sample. The data for fold change was analyzed using 2−ΔΔCt 14,16. For example, the ΔΔCt for Smad3 mRNA expression in GU group in 14-day was as follow: ΔΔCtSmad3, 14-day, GU group = (CTSmad3, 14-day, GU group—CTβ-actin, 14-day, GU group)—(CTSmad3, 14-day, SHO group—CTβ-actin, 14-day, SHO group), and the fold change for Smad3 mRNA expression in GU group in 14-day was 2−ΔΔCtSmad3, 14-day, GU group.
Western-Blot Analysis
The total proteins of renal tissue were extracted using lysing buffer (Beyotime Institute of Biotechnology, Haimen, China), following a centrifugation at 12,000×g for 10 min at 4°C, and protein content in the supernatant was detected using the BCA protein assay. Equal amounts of extract (30 μg) were then separated on an 8% sodium dodecylsulfate (SDS)-poly-acrylamide gel and transferred by electroblotting to PVDF membranes. The membranes were incubated in 5% skimmed milk for 1 h and were washed with 0.1% TBST at room temperature, and followed by overnight incubation at 4°C with primary antibodies: anti-Smad3 (1:5000, Santa Cruz Biotechnology) and anti-β-actin (1:5000, Epit Mics). Primary antibodies were diluted using 0.1% TBST. Alkaline phosphatase conjugated secondary antibodies were incubated with blots for 1 h at room temperature. After washing, blots were developed by the ECL Western Blotting detection system, and then exposed to X-ray film for visualization of the protein bands, and membranes were semi-quantified using the Quantity One image analysis system (4.3.1, Bio-Rad, Inc., Hercules, CA, USA). The expression level of Smad3 was corrected by comparison with β-actin.
STATISTICAL ANALYSIS
The data are shown as mean ± standard deviation (SD). To compare the groups in relation to parameters with normal distribution, One-way analysis of variation (ANOVA) with post-hoc Fisher’s LSD (least significant difference) was used. For parameters without normal distribution, Kruskal-Wallis with post-hoc Mann-Whitney (only for the weight parameter) was used.Citation17,18 Pearson’s correlation coefficients were used to determine the relationships between the indicators for detection. A value of p < 0.05 was accepted as statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS, version 13.0; Chicago, IL, USA).
RESULTS
Renal Morphology
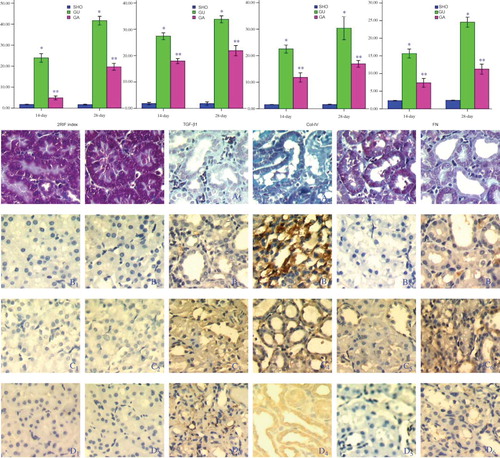
Light microscope showed more collagen deposition, fibroblast proliferation and diffused lymphocytes filtration in the renal tubulointerstitial of GU group (: A3 and A4), but the lesion degree in GA group was less serious than that in GU group (: A5 and A6). RIF index in GU group was notably elevated when compared with that in SHO (p < 0.01; ). However, the index of RIF in GA group was markedly decreased when compared with that in GU group (p < 0.01; ).
Figure 1. (1) Statistical parameters in three groups. (2) Tissue characteristics of renal interstitium in three groups. Masson staining for SHO group (A1, 14-day and A2, 28-day), GU group (A3, 14-day and A4, 28-day) and GU group (A5, 14-day and A6, 28-day). Representative samples of immunohistochemical staining for TGF-β1 (SHO: B1, 14-day and B2, 28-day, GU: B3,14-day and B4, 28-day; and GA B5,14-day and B6, 28-day), Col-IV (SHO: C1, 14-day and C2, 28-day and GU: C3,14-day and C4: 28-day; GA: C5,14-day and C6, 28-day) and FN (SHO: D1, 14-day and D2, 28-day, GU: D3,14-day and D4, 28-day; and GA: D5,14-day and D6, 28-day) were observed in all groups. Positive stainings for collagen fibers (blue, masson’s trichrome staining) in GU group was notably marked than that in SHO group and ATRA group. Positive staining for TGF-β1, Col-IV or FN was strong in GU group when compared with that in SHO. The positive stainings in ATRA group were remarkably reduced when compared with those in GS group. TGF-β1, transforming growth factor-β1; Col-IV, collagen IV; FN, fibronectin; ATRA, all-trans retinoic acid; SHO, sham operation group; GU, model group subjected to unilateral ureteral obstruction, GA, model group treated with ATRA. Magnification 400x.Note: *p < 0.01 compared with SHO, **p < 0.01 compared with GU.
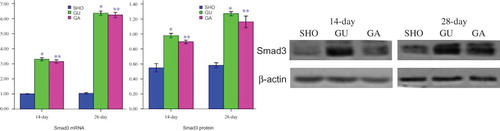
Effect of ATRA on TGF-β1, Col-IV or FN Protein Expression
Comparison with those in SHO group, in GU group, the protein expressions of TGF-β1, Col-IV and FN in the tubulointerstitial were significantly increased (all p < 0.01; ). However, the TGF-β1, Col-IV and FN expressions in ATRA treatment group were lower than those in GU group (each p < 0.01; ).
Effect of ATRA on the mRNA and Protein Expressions of Smad3
When compared with those in SHO group, renal tissue of GU group showed consistently higher Smad3 mRNA and protein expressions (all p < 0.01; ). However, the Smad3 mRNA and protein expressions were not markedly reduced in GA group when compared with those of model group (all p > 0.05; ).
Correlation Analysis
Protein expression of Smad3 was positively correlated with index of RIF, protein expression of TGF-β1, Col-IV or FN (r = 0.646, 0.759, 0.687, 0.763; each p < 0.05).
DISCUSSION
In this study, we found that index of RIF, protein expression of TGF-β1, Col-IV or FN, were markedly increased in GU group than those in SHO group, especially in 28-day. Furthermore, the expressions of mRNA and protein of Smad3 in GU group were notably increased when compared with those in group SHO, especially in 28-day. TGF-β1/Smad3 signaling pathway took part in the pathogenesis of renal fibrosis in this study. This result was similar with that from some other reports.Citation19–21 TGF-β1/Smad3 signaling is a most important pathway leading to renal fibrosis.
ATRA, as a critical signaling molecule, regulates a lot of transcription factor. There were some studies which reported that ATRA could play a protective role in the prevention of renal diseases. Our previous study Citation14 found that the ATRA could alleviate the glomerulosclerosis lesion in rats. Kishimoto et al. conducted an interesting investigation and reported that ATRA had a preventive effect on renal fibrosis induced by UUO.Citation4 So, ATRA could play a protective role against the renal diseases. Its result was similar with that in our study. However, whether there was an association between ATRA and TGF-β1/Smad3 signaling pathway in the pathogenesis of renal fibrosis, there was no any report. This investigation was performed to explore this association. In our study, we found that the ATRA could play a protective role against RIF but could not regulate the TGF-β1/Smad3 signaling pathway.
We reviewed those studies reported the relationship between ATRA and TGF-β1/Smad3 signaling pathway. There was no any study reporting the association between ATRA and TGF-β1/Smad3 signaling pathway in the pathogenesis of renal fibrosis. However, some investigator found that there was an association between ATRA and TGF-β/Smad signaling pathway. Yu et al.Citation22 found that ATRA treatment significantly increased the level of TGF-β3 in comparison to level in untreated mouse embryonic palate mesenchymal micromass cultures, and ATRA inhibited the phosphorylation of Smad3, and they drew a conclusion that ATRA-induced hypochondrogenesis through regulation of the TGF-β3 pathway. Zhang et al.Citation23 performed a further study in mouse embryonic palate mesenchymal cells (MMCs) and the results were as follows: ATRA inhibited chondrogenesis and the levels of phospho-Smad2/3; whereas, treatment with TGF-β3 strongly induced phospho-Smad2/3 and chondrogenesis. Additionally, combined treatment with ATRA plus TGF-β3 significantly reduced the levels of phospho-Smad2/3 and repressed chondrogenesis compared with that seen in MMCs treated with TGF-β3 alone. They drew a conclusion that ATRA was able to suppress TGF-β3-induced Smad2/3 phosphorylation and chondrogenesis.
As those mentioned above, our study was the first report to investigate the association between ATRA and TGF-β1/Smad3 signaling pathway. Unfortunately, we could not find the association. We checked our data carefully again and found that the results were robust. We speculated that the ATRA might not play a protective role by TGF-β1/Smad3 signaling pathway, and the ATRA might regulate other Smads expression to take the role of prevention from RIF but not Smad3, such as Smad4.Citation24 Furthermore, ATRA could reduce the expressions of various factors which could induce renal diseases, such as inducible nitric oxide synthase,Citation25 monocyte chemotactic protein-1,Citation4 plasminogen activator inhibitor-1Citation26 and so on.
In conclusion, ATRA treatment can reduce the expression of TGF-β1 and alleviate the RIF progression in UUO rats. However, ATRA cannot affect the TGF-β/Smad signaling pathway in the progression of RIF. More studies are needed to explore the other possible signal pathways and molecular mechanisms, and confirm the role of ATRA in the pathogenesis of RIF in future.
ACKNOWLEDGMENTS
This study was supported by the Nature Science Foundation of China (no. 81060061), the Natural Science Foundation of the Guangxi Zhuang Autonomous Region (no. 0832121) and the Health Department of Guangxi Zhuang Autonomous Region (no. 200917). The authors would like to gratefully acknowledge the most helpful comments on this article received from Professor Liang Rong, Department of Pediatric-Neonatology, Baylor College of Medicine, Houston, Texas, USA.
Conflicts of Interest: The authors declare that they have no conflict of interest.
Author contributions: Tian-Biao Zhou and Yuan-Han Qin conceived and designed the experiments; Zheng-Yi Li, Li-Na Su, Tian-Biao Zhou, Yuan-Han Qin, and Hui-Ling Xu performed the experiments; Tian-Biao Zhou, Li-Na Su, and Feng-Ying Lei analyzed the data; Yuan-Han Qin contributed reagents/materials/analysis tools; and Zheng-Yi Li and Tian-Biao Zhou wrote the paper.
REFERENCES
- Thatcher JE, Zelter A, Isoherranen N. The relative importance of CYP26A1 in hepatic clearance of all-trans retinoic acid. Biochem Pharmacol. 2010;80:903–912.
- Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. 2012;1821:152–167.
- Mauney JR, Ramachandran A, Yu RN, Daley GQ, Adam RM, Estrada CR. All-trans retinoic acid directs urothelial specification of murine embryonic stem cells via GATA4/6 signaling mechanisms. PLoS One. 2010;5:e11513.
- Kishimoto K, Kinoshita K, Hino S, . Therapeutic effect of retinoic acid on unilateral ureteral obstruction model. Nephron Exp Nephrol. 2011;118:e69–e78.
- Tabata C, Tabata R, Nakano T. The calpain inhibitor copeptin prevents bleomycin-induced pulmonary fibrosis in mice. Clin Exp Immunol. 2010;162:560–567.
- Wang H, Dan Z, Jiang H. Effect of all-trans retinoic acid on liver fibrosis induced by common bile duct ligation in rats. J Huazhong Univ Sci Technolog Med Sci. 2008;28:553–557.
- Kawakatsu M, Kanno S, Gui T, . Loss of Smad3 gives rise to poor soft callus formation and accelerates early fracture healing. Exp Mol Pathol. 2011;90:107–115.
- Chen HY, Huang XR, Wang W, . The protective role of Smad7 in diabetic kidney disease: Mechanism and therapeutic potential. Diabetes. 2011;60:590–601.
- Latella G, Vetuschi A, Sferra R, . Targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice. Liver Int. 2009;29:997–1009.
- Lan HY. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–1067.
- Zhou TB, Qin YH, Zhou C, . Less expression of prohibitin is associated with the increased Caspase-3 expression and cell apoptosis in renal interstitial fibrosis rats. Nephrology (Carlton). 2012;17:189–196.
- Zhou TB, Qin YH, Ou C, . All-trans retinoic acid can regulate the expressions of gelatinases and apolipoprotein E in glomerulosclerosis rats. Vascul Pharmacol. 2011;55:169–177.
- Radford MG Jr, Donadio JV Jr, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207.
- Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. All-trans retinoic acid regulates the expression of apolipoprotein E in rats with glomerulosclerosis induced by Adriamycin. Exp Mol Pathol. 2011;90:287–294.
- Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ, Huang WF. apoE expression in glomerulus and correlation with glomerulosclerosis induced by adriamycin in rats. Ren Fail. 2011; 33:348–354.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408.
- Cury JL, Brunetto AF, Aydos RD. Negative effects of chronic kidney failure on lung function and functional capacity. Rev Bras Fisioter. 2010;14:91–98.
- Piroozmand A, Hassan ZM. Evaluation of natural killer cell activity in pre and post treated breast cancer patients. J Cancer Res Ther. 2010;6:478–481.
- Qin W, Chung AC, Huang XR, . TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–1474.
- Chung AC, Huang XR, Zhou L, Heuchel R, Lai KN, Lan HY. Disruption of the Smad7 gene promotes renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) in mice. Nephrol Dial Transplant. 2009;24:1443–1454.
- Zhang D, Sun L, Xian W, . Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/Smad activity. Lab Invest. 2010;90:436–447.
- Yu Z, Xing Y. All-trans retinoic acid inhibited chondrogenesis of mouse embryonic palate mesenchymal cells by down-regulation of TGF-beta/Smad signaling. Biochem Biophys Res Commun. 2006;340:929–934.
- Zhang H, Li N, Tang Y, Wu W, Zhang Q, Yu Z. Negative functional interaction of retinoic acid and TGF-beta signaling mediated by TG-interacting factor during chondrogenesis. Cell Physiol Biochem. 2009;23:157–164.
- Zhong H, Wang HR, Yang S, . Targeting Smad4 links microRNA-146a to the TGF-beta pathway during retinoid acid induction in acute promyelocytic leukemia cell line. Int J Hematol. 2010;92:129–135.
- Datta PK, Lianos EA. Retinoic acids inhibit inducible nitric oxide synthase expression in mesangial cells. Kidney Int. 1999;56:486–493.
- Liu X, Lu L, Tao BB, Zhu YC. All-trans retinoic acid inhibits the increases in fibronectin and PAI-1 induced by TGF-beta1 and Ang II in rat mesangial cells. Acta Pharmacol Sin. 2008;29:1035–1041.