Abstract
Background: Left ventricular hypertrophy (LVH) and systolic dysfunction would predict the mortality of patients undergoing maintenance hemodialysis. The cause of LVH is usually related to the increase of total peripheral vascular resistance and overloading volume. The presence of residual diuresis enables greater control of the volume. This study evaluated the effects of residual renal function (RRF) on the left ventricle and analyzed the related factors involved in hemodialysis patients. Methods: A total of 59 hemodialysis patients were classified into two groups. The patients in the RRF (RRF+) group had a urine volume greater than 200 mL/24 h, and the patients in the non-RRF (RRF−) group had a urine volume less than 200 mL/24 h. B-type natriuretic peptide (BNP), blood total homocysteine (tHcy), and blood biochemical indexes were determined for the patients in both groups. Echocardiography and Doppler tests were performed to determine the cardiac indexes. Results: LVH and systolic dysfunction in the RRF+ group were less severe than those in the RRF− group. The concentration of tHcy and BNP in patients with RRF was decreased in comparison with those without RRF. The concentration of tHcy and BNP was positively correlated with the residual diuresis. Conclusions: There were distinct ventricular geometric patterns and different functional performances between RRF+ and RRF− groups. The presence of residual diuresis had a beneficial effect on the left ventricular function in hemodialysis patients.
INTRODUCTION
Residual renal function (RRF) is recognized as an important factor influencing morbidity, mortality, and quality of life in chronic dialysis patients.Citation1–3 During the conservative management of patients undergoing maintenance hemodialysis (MHD), the decrease of the glomerular filtration rate (GFR) was associated with left ventricular hypertrophy (LVH).Citation4–7 The presence of LVH, in turn, constitutes an important independent factor of cardiovascular risk in patients with chronic kidney disease (CKD), including MHD patients. Both LVH and systolic dysfunction have major contributions to this increased risk of cardiovascular death.Citation4,5,8
Many studies have been carried out to discover useful prognostic markers for MHD patients. For instance, blood total homocysteine (tHcy)Citation9–13 and B-type natriuretic peptide (BNP)Citation14–17 have been shown to have good predictive power for the total and cardiovascular disease (CVD) mortality in patients with end-stage renal disease (ESRD). Both tHcy and BNP are useful in identifying LVH or depressed left ventricular systolic function in asymptomatic ESRD patients.
Various studies have shown that the presence of LVH was related to hemodialysis (HD) treatment. However, there was no clear evidence of the relationship between LVH and the function of the left ventricle or the biomarkers with different volumes of residual diuresis. Therefore, the objectives of this study were to verify possible effects of RRF on the left ventricular structure and function and analyze the relationship of tHcy and BNP with chronic hemodialysis treatment.
PATIENTS AND METHODS
Patients
Between June 2006 and December 2010, 59 MHD patients who had been on hemodialysis for at least 6 months in Renmin Hospital of Wuhan University were enrolled into the study. The study protocol was reviewed and approved by an independent ethics committee and all participants gave written informed consent. All patients who received regular hemodialysis treatment and agreed to participate in the study met the inclusion criteria. Patients with a previous clinical history of CVD, diabetes, and uncontrolled blood pressure were excluded from this study. Using echocardiogram, those patients with an ejection fraction less than 55% and with segmental changes in left ventricular contractility were also excluded. All patients received regular dialysis using polysulfone dialyzer three times per week, each lasting 4 h. The dialysate was bicarbonate buffered, the dialysate flow rate was 500 mL/min, and blood flow ranged from 250 to 300 mL/min. Fifty nine patients were divided into two groups. The patients (n = 27) in the RRF (RRF+) group had a urine volume greater than 200 mL/24 h, and the patients (n = 32) in the non-RRF (RRF−) group had a urine volume less than 200 mL/24 h. The following clinical and laboratory tests including systolic arterial pressure (SAP), diastolic arterial pressure (DAP), length of hemodialysis treatment, urinary 24-h volume (UV24 h), hemoglobin (Hb), serum calcium (Ca), serum phosphate (P), parathormone (PTH), serum albumin (Alb), tHcy, and BNP were performed for the patients in both groups.
Methods
SAP and DAP were immediately obtained in the morning between 8 and 9 am before the HD session using the arm opposite to the arteriovenous (AV) fistula and represented the average of the last 10 HD sessions. Mean arterial blood pressure (MAP) was calculated using the formula: MAP= DAP + (SAP−DAP/3). The UV24 h (mL) was collected during the interdialytic period. Interdialytic weight gain (iWG) represents the difference between body weight immediately after the HD session and the weight immediately before the next HD session. The iWG value was considered as the arithmetic average of the last 10 HD sessions. The Kt/V index was used to assess the adequacy of dialysis. The effective clearance plasma Kt (K: urea clearance, t: effective dialysis duration) was delivered by automatic measurements and calculations. The Watson formula was used to calculate body fluid volume. All blood samples were taken at the start of the HD session from the middle of the week. Plasma tHcy was determined using high-performance liquid chromatography assay (HP 1100, Agilent Technologies, MN, USA). BNP was measured with the TRIAGE BNP kit (Biosite Diagnostics, San Diego, CA, USA) according to the guidelines supplied by the manufacturer. PTH values were determined using the immunoradiometric method and normal values were considered to be between 10 and 65 pg/mL. Other biochemical tests were performed by standard laboratory methods described previously. Echocardiography was performed using a GE-VingMed System 5 echocardiographic machine (GE-VingMed Sound AB, Horten, Norway) with a 3.3-mHz multiphase array probe in subjects lying in the left decubitus position by a single experienced examiner blinded to all of the clinical details after a midweek HD. The Doppler echocardiographic variables were analyzed according to the criteria from the American Society of Echocardiography.Citation18 Interventricular septal diameter (IVSd) and posterior wall diastolic diameter (PWDd) were measured. The mean wall thickness (MWT) was calculated using the following formula: MWT (mm) = (IVS + PWT)/2, where IVS represents the interventricular septum and PWT represents the posterior wall thickness. The left ventricular end-diastolic diameter (LVEDd) was assessed in late diastolic phase. The relative wall thickness (RWT) was calculated using the formula: RWT = PWDd + IVSd/LVEDd, where IVSd represents the IVS thickness in diastole. The left ventricular mass (LVM) was measured using the Devereux and Reichek formula: LVM (g) = 1.04 × ([LVId + IVSd + PWT]3 − LVId3) − 13.6 g, where LVId represents the left ventricular internal diameter. The LVM index (LVMI) was calculated as the LVM to body surface area ratio and expressed as g/m2. The following parameters were also evaluated: left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), end-systolic diameter of the left ventricle (LVESd), end-diastolic volume index, and end-systolic volume index of the left ventricle.
Statistical Analysis
For the statistical analysis, we conducted t-tests to compare the averages between the groups. Pearson’s correlation coefficient was calculated. The data was expressed as means ± SD. Statistical significance was set at p lower than 0.05 for a two-tailed t-test or χ2-test, where appropriate. Data analysis was performed using the SPSS l3.0.
RESULTS
Clinical and laboratory characteristics of patients in both groups are presented in and . Detailed echocardiographic data are provided in .
Table 1. Clinical characteristics of the patients.
Table 2. Laboratory parameters.
Table 3. Echocardiographic parameters.
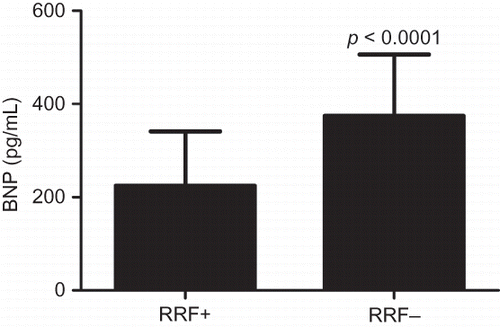
The concentration of tHcy and BNP in the patients of both groups is shown in and . The results showed that tHcy and BNP in the RRF+ group were significantly lower than those in the RRF− group (p < 0.0001).
Figure 1. tHcy in RRF+ and RRF− groups. tHcy in the RRF+ group was significantly lower than that in the RRF− group.
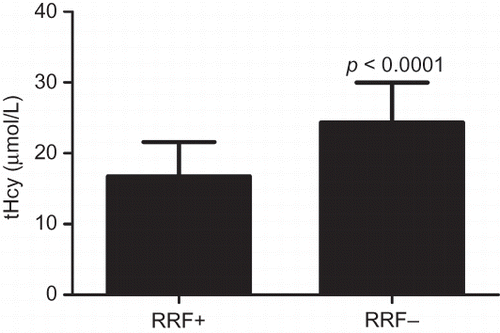
Figure 2. BNP in RRF+ and RRF− groups. BNP in the RRF+ group was significantly lower than that in the RRF− group.
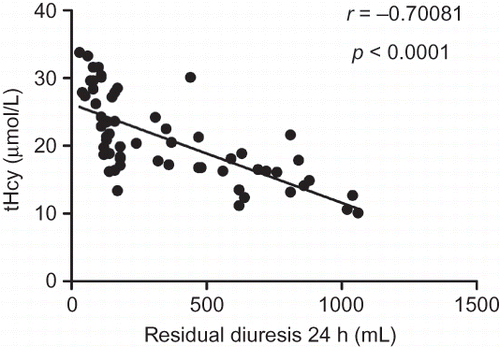
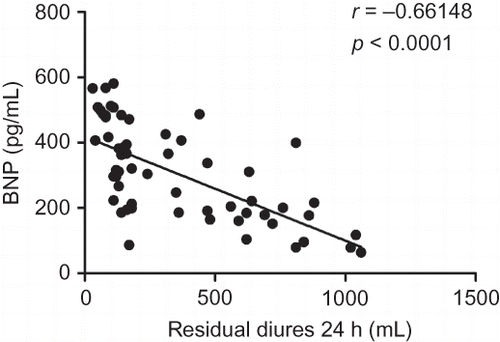
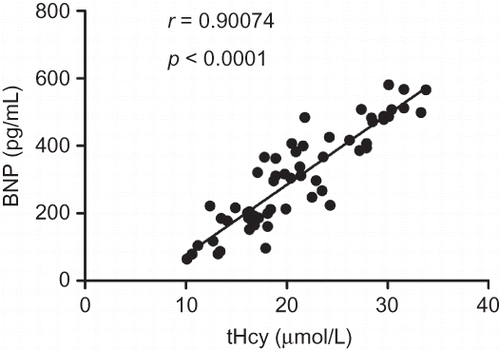
The correlations of tHcy and BNP with residual diuresis are presented in . Both tHcy (r = −0.70081, p < 0.0001) and BNP (r = −0.66148, p < 0.0001) were correlated with residual diuresis. tHcy strongly correlated with BNP (r = 0.90074, p < 0.0001).
DISCUSSION
RRF refers to the small renal function activity persisting in patients affected by chronic renal failure in the uremic phase.Citation19 RRF not only improves the nutritional status and quality of life, but also reduces the mortality in patients with chronic dialysis.Citation20 RRF is evaluated by the mean of creatinine and urea clearance rate and corrected with the body surface area. Detection of creatinine and urea clearance is a heavy burden for chronic dialysis patients because it requires collection of urine for 24 h. Therefore, some studies used the dialysis between 24 h urine to evaluate RRF.Citation1,21,22 Moist et al.Citation22 showed that the urine of hemodialysis patients was positively correlated with the inulin clearance rate (correlation coefficient: 0.71) and the correlation coefficient of urine for 24 h with the mean of creatinine and urea clearance was 0.57 (p = 0.001). Van Olden et al.Citation23 also observed a significant positive correlation between GFR and urine volume for 24 h. These results support that 24 h urine volume is an effective indicator for the evaluation of RRF. Other studies also used this indicator to define RRF loss (24 h urine < 200 mL).Citation21,22
Under the hemodialysis condition, it is required to clear 60–70 L plasma water weekly in order to ensure the removal of the body’s toxin. Studies have shown that the RRF is 1 mL/min, which is equivalent to the weekly removal of approximately 10 L of plasma water. Therefore, under hemodialysis condition, patients with RRF are easier to achieve fluid balance than those without RRF. Furthermore, blood pressure can be better controlled in the patients with RRF.
In general, very little RRF can effectively control blood volume to minimize the effect of dialysis. In addition, RRF can produce a certain amount of erythropoietin and active vit D3 and promote hematopoietic function, which maximizes and maintains the calcium and phosphorus balance and actively improves the anemia and nutritional status. In our study, hemoglobin and serum calcium in RRF+ group were higher than those in RRF− group, while serum phosphate and parathormone were higher in RRF− group. These results confirmed the effect of RRF on endocrine system.
In the general population, cardiac disease is usually caused by atheromatous lesions in the coronary arteries. However, among MHD patients, the main cardiac abnormality is LVH, accounting for 75% of cardiac abnormality.Citation24 Epidemiological studies have consistently demonstrated that CVD is the main cause of mortality in HD patients and is partly attributed to the high prevalence of accelerated atherosclerosis and LVH. For patients with long-term dialysis therapy, LVH was frequently complicated with LV dilatation and systolic dysfunction.Citation24 Furthermore, progressive worsening of cardiac dilatation with compensatory hypertrophy occurred with the extension of time on dialysis.Citation25 Wang et al.Citation26 observed that cardiovascular events in patients with RRF were significantly higher than those without RRF. Araujo et al.Citation27 proved that preservation of diuresis can influence LVM and function in patients with chronic kidney failure. Our study indicated that LVH was significantly reduced and left ventricular function was improved in RRF+ group than in RRF− group. These results suggest that RRF has a certain protective effect on the left ventricular structure and function in MHD patients. Hemoglobin, PTH, and mean arterial pressure may account for this effect.
Homocysteine is an intermediate sulfur-containing amino acid formed by the conversion of methionine, an essential amino acid, into cysteine. Hyperhomocysteinemia (Hhcy) occurs in about 85% of CKD patients because of the altered renal metabolism and impaired renal excretion.Citation28,29 Many studies show that Hhcy is an independent cardiovascular risk factor,Citation30,31 and it probably plays a causative role in the atherothrombotic process.Citation32,33 Thus, tHcy is considered by some researchers as a marker rather than a risk factor for cardiovascular events. Many factors influence the level of tHcy concentration, but the relationship between tHcy and RRF is unknown. We demonstrated that tHcy had a negative correlation with residual diuresis in MHD patients.
A significant increase of BNP concentration has been observed in patients with chronic dialysis. BNP is secreted by ventricular cardiomyocytes in response to the increased cardiac strain and correlates with hypervolemia and mortality in HD patients.Citation34 It has also been shown that BNP has a good predictive power for the total and CVD mortality in patients with ESRD.Citation14–17,35 Mallamaci et al.Citation16 also showed that cardiac natriuretic peptides, particularly BNP, had a high sensitivity in diagnosing LVH and systolic dysfunction in patients with dialysis. In our study, we found that BNP had a negative correlation with residual diuresis in MHD patients.
These results indicated that patients with RRF have less severe LVH and better left ventricular function, but also have a lower concentration of tHcy and BNP than those without RRF.
Although tHcy and BNP are useful markers in identifying LVH or depressed left ventricular systolic function in asymptomatic ESRD patients, few studies investigated the relationship between tHcy and BNP in MHD patients. Herrmann et al.Citation36 found that cardiac BNP expression was significantly induced by chronically increased tHcy concentrations in rats. We showed a significant positive relation between tHcy and BNP in MHD patients.
In conclusion, our data suggest that RRF plays an important role in the maintenance of patients with hemodialysis. The preservation of diuresis appears to be beneficial for LMV and function in patients with chronic kidney failure. BNP and tHcy have good predictive power for left ventricular function in these patients.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Merkus MP, Jager KJ, Dekker FW, Boeschoten EW, Stevens P, Krediet RT. Quality of life in patients on chronic dialysis: self-assessment 3 months after the start of treatment. The NECOSAD Study Group. Am J Kidney Dis. 1997;29:584–592.
- Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT. Predictors of poor outcome in chronic dialysis patients: The Netherlands Cooperative Study on the Adequacy of Dialysis. The NECOSAD Study Group. Am J Kidney Dis. 2000;35:69–79.
- Shemin D, Bostom AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38:85–90.
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5:2024–2031.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566.
- Parfrey PS, Harnett JD, Griffiths SM, . The clinical course of left ventricular hypertrophy in dialysis patients. Nephron. 1990;55:114–120.
- Gunal AI, Kirciman E, Guler M, Yavuzkir M, Celiker H. Should the preservation of residual renal function cost volume overload and its consequence left ventricular hypertrophy in new hemodialysis patients? Ren Fail. 2004;26:405–409.
- Verdecchia P, Carini G, Circo A, . Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol. 2001;38:1829–1835.
- Bachmann J, Tepel M, Raidt H, . Hyperhomocysteinemia and the risk of vascular disease in hemodialysis patients. J Am Soc Nephrol. 1995;6:121–125.
- Moustapha A, Naso A, Nahlawi M, . Prospective study of hyperhomocysteinemia as an adverse cardiovascular risk factor in end-stage renal disease. Circulation. 1998;97:138–141.
- Robinson K, Gupta A, Dennis V, . Hyperhomocysteinemia confers an independent increased risk of atherosclerosis in end-stage renal disease and is closely linked to plasma folate and pyridoxine concentrations. Circulation. 1996;94:2743–2748.
- Rossi GP, Seccia TM, Pessina AC. Homocysteine, left ventricular dysfunction and coronary artery disease: is there a link? Clin Chem Lab Med. 2007;45:1645–1651.
- Cesari M, Zanchetta M, Burlina A, . Hyperhomocysteinemia is inversely related with left ventricular ejection fraction and predicts cardiovascular mortality in high-risk coronary artery disease hypertensives. Arterioscler Thromb Vasc Biol. 2005;25:115–121.
- Zoccali C, Mallamaci F, Benedetto FA, . Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001;12:1508–1515.
- Naganuma T, Sugimura K, Wada S, . The prognostic role of brain natriuretic peptides in hemodialysis patients. Am J Nephrol. 2002;22:437–444.
- Mallamaci F, Zoccali C, Tripepi G, . Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int. 2001;59:1559–1566.
- Sanjuan R, Oliva SM, Blasco ML, . Plasma brain natriuretic peptide levels in cardiac function assessment in chronic dialysis patients. Cardiorenal Med. 2011;1:147–155.
- Schiller NB, Shah PM, Crawford M, . Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367.
- Carofei O, Galeotti P, Satriani M, Pedulla F, Taratufolo A. Clinical impact of residual function in hemodialysis. EDTNA ERCA J 1997; 23:54–56.
- Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K. Residual renal function improves outcome in incremental hemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009;24:2502–2510.
- Suda T, Hiroshige K, Ohta T, . The contribution of residual renal function to overall nutritional status in chronic hemodialysis patients. Nephrol Dial Transplant. 2000;15:396–401.
- Moist LM, Port FK, Orzol SM, . Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564.
- Van Olden RW, van Acker BA, Koomen GC, Krediet RT, Arisz L. Time course of inulin and creatinine clearance in the interval between two hemodialysis treatments. Nephrol Dial Transplant. 1995;10:2274–2280.
- Foley RN, Parfrey PS, Harnett JD, . Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192.
- Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Long-term evolution of cardiomyopathy in dialysis patients. Kidney Int. 1998;54:1720–1725.
- Wang AY, Wang M, Lam CW, Chan IH, Zhang Y, Sanderson JE. Left ventricular filling pressure by Doppler echocardiography in patients with end-stage renal disease. Hypertension. 2008;52:107–114.
- Araujo S, Lemes HP, Cunha DA, Queiroz VS, Nascimento DD, Ferreira Filho SR. Cardiac morphology and function in patients with and without residual diuresis on hemodialysis. J Bras Nefrol. 2011;33:74–81.
- De Koning L, Hu FB. Homocysteine lowering in end-stage renal disease: is there any cardiovascular benefit? Circulation. 2010;121:1379–1381.
- Anan F, Masaki T, Umeno Y, . Correlations between homocysteine levels and atherosclerosis in Japanese type 2 diabetic patients. Metabolism. 2007;56:1390–1395.
- Kendrick J, Chonchol MB. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol. 2008;4:672–681.
- Anan F, Takahashi N, Shimomura T, . Hyperhomocysteinemia is a significant risk factor for silent cerebral infarction in patients with chronic renal failure undergoing hemodialysis. Metabolism. 2006;55:656–661.
- Tawakol A, Omland T, Gerhard M, Wu JT, Creager MA. Hyperhomocyst(e)inemia is associated with impaired endothelium- dependent vasodilation in humans. Circulation. 1997;95: 1119–1121.
- Wang H, Yoshizumi M, Lai K, . Inhibition of growth and p21ras methylation in vascular endothelial cells by homocysteine but not cysteine. J Biol Chem. 1997;272: 25380–25385.
- Hörl WH. Natriuretic peptides in acute and chronic kidney disease and during renal replacement therapy. J Investig Med. 2005;53:366–370.
- Collado S, Coll E, Deulofeu R, . Prevalence of cardiovascular disease in uraemia and relevance of cardiovascular risk factors. Nefrologia. 2010;30:342–348.
- Herrmann M, Taban-Shoma O, Hübner U, . Hyperhomocysteinemia and myocardial expression of brain natriuretic peptide in rats. Clin Chem. 2007;53:773–780.