Abstract
Patients on hemodialysis (HD) show changes in muscle structure and function reducing their functional capacity. This study was conduted to assess the effects of respiratory muscle training (RMT) and peripheral muscle training (PMT) during dialysis on functional parameters, inflammatory state, and quality of life (QoL) in patients on HD. Randomized controlled trial included 39 patients on HD, and they were divided into three groups: RMT (n = 11), PMT (n = 14), and controls (C, n = 14). Training was performed during the HD session for 10 weeks. Maximal inspiratory pressure (PImax), maximal expiratory pressure (PEmax), forced vital capacity (FVC), six-minute walk test (6MWT), Kt/Vsp, biochemical parameters, and inflammatory state (i.e., level of high sensitivity C-reactive protein) were evaluated. Variation from baseline was calculated by Analysis of Covariance (ANCOVA). The ΔPImax was 22.5 ± 3.2, 9.1 ± 2.9, and −4.9 ± 2.8 cmH2O in the RMT, PMT and C, respectively (p < 0.001); ΔPEmax was 10.8 ± 6.6, 3.7 ± 5.9, and −15.6 ± 5.9 cmH2O respectively (p = 0.014). The Δ6MWT was significantly greater in RMT and PMT (65.5 ± 9; 30.8 ± 8 m) than in C (−0.5 ± 8.1 m), p < 0.001. Although biochemical parameters decreased after training, Kt/V remained unchanged. CRP decreased only in the RMT and PMT groups. There was a significant increase in QoL scores in the training groups (vs. C) in energy/fatigue (p = 0.002), sleep (p < 0.001), pain (p < 0.001), and list of symptoms/problems (p = 0.014). A short period of RMT or PMT during HD significantly improved functional capacity, with RMT showing greater effect than PMT. Muscle training improved biochemical and inflammatory markers, but a direct cause and effect relationship could not be established by this study.
INTRODUCTION
Patients with chronic kidney disease (CKD) show changes in muscle structure and function. The progression of CKD leads to the development of uremic myopathy which is characterized by muscle wasting and reduction of physical capacity.Citation1–4 Both CKD and hemodialysis (HD) themselves decrease respiratory and peripheral muscle strength, further worsening the functional loss in these patients.Citation3 Additionally, the muscle impairment significantly affects the quality of life (QoL), causing fatigue, pain, mobility restriction, and psychological changes.Citation4
There is evidence that muscle training administered to these patients during HD sessions can change this scenario, promoting a significant improvement in their physical and functional capacity.Citation4,5 The functional capacity, respiratory, and peripheral muscle strength can be measured before and after muscle training using tests such as the six-minute walk test (6MWT), the respiratory pressure measurement, and the maximum strength test respectively.Citation3,5 Also, there is speculation that physical exercise increases HD efficiency, in addition to reducing inflammation, oxidative stress, and positively influencing QoL parameters.Citation4–7
The benefits of muscle training during HD or in the period between dialysis sessions have been studied, most of them use aerobic training programs.Citation4,8 The scarcity of studies assessing strength training in patients with end-stage renal disease (ESRD) and their conflicting resultsCitation9 motivated us to conduct this study to assess the effects of respiratory and peripheral muscle training on functional capacity, HD efficiency, inflammatory state, and QoL of patients with ESRD.
METHODS
Study Design
This is a randomized controlled clinical trial aiming to study respiratory and peripheral muscle training, and having as endpoints changes in functional, biochemical, and inflammatory parameters.
Patients and Randomization
Forty-five patients with ESRD on HD at the Dialysis Unit of Santa Casa de Misericórdia, Porto Alegre, Rio Grande do Sul were enrolled from June to September 2009. The inclusion criteria were the following: age between 18 and 70 years, to be on dialysis for more than 3 months, and to agree to participate by signing an informed consent form. Exclusion criteria were the following: patients with unstable angina, uncontrolled cardiac arrhythmia, decompensated heart failure, systolic blood pressure (BP) greater than 200 mmHg, diastolic BP greater than 120 mmHg, acute pericarditis or myocarditis, decompensated diabetes mellitus (fasting serum glucose greater than 300 mg/dL), severe untreated mitral or aortic insufficiency/stenosis, severe lung conditions, acute systemic infection, severe bone disease, patients with lower limb amputations, patients with cognitive disorders, and those who are unable to perform the proposed tests due to disabling musculoskeletal, bone, or joint disorders.
Randomization was made by dividing the subjects into three blocks of 15 each, five in each group, for one of the following: Respiratory Muscle Training (RMT), Peripheral Muscle Training (PMT), or Control (C).
The study was approved by the Ethical Review Board of Irmandade da Santa Casa de Misericórdia, de Porto Alegre, under protocol number 3087/09, in adherence with the Declaration of Helsinky.
HD Procedure
Thrice-weekly HD was performed in a Tina machine (Baxter, Derfield, IL, USA), with capillary filter size 10 L (Gambro, Stockholm, Sweden). The standard prescription for the HD was blood flow rate at 300 mL/min, dialysate flow rate at 700 mL/min, and total dialysis session length of 4 h. Vascular access was through an arteriovenous fistula in all patients.
Test Procedures
The assessment protocol included history-taking to collect demographic information, baseline and previous diseases, use of medications, time on HD, smoking, alcohol use, weight (W), height (H), and body mass index (BMI, calculated through the W/H2 ratio). The assessment of respiratory muscle strength and function included (a) measurement of maximal inspiratory pressure (PImax) and maximal expiratory pressure (PEmax) through a respiratory pressure meter; (b) measurement of forced vital capacity (FVC) by spirometry; and (c) assessment of functional capacity by the 6MWT. The instrument used to assess QoL was the Kidney Disease and Quality of Life Short Form (KDQOL-SF™1.3). Blood was drawn for biochemical tests and to measure the inflammatory state before dialysis session. All functional tests were performed on different days—non-dialysis days–—to avoid interference in test results. All tests were performed before training (baseline) and after 70 days.
Measurement of respiratory muscle strength
Respiratory muscle strength was measured with a respiratory pressure meter (MVD300®, Globalmed, Porto Alegre, Brazil) that can measure positive and negative pressures within the range of −300 and +300 cmH2O from the measurement of PImax and PEmax. Both parameters were recorded according to the criteria set by the Brazilian Pneumology and Tisiology Society.Citation10 The greatest value recorded (in cmH2O) was selected for each of the pressures and compared to reference values proposed by Neder et al.,Citation11 which predicts the expected values for the Brazilian population according to gender and age.
Spirometry
FVC was measured with a handheld spirometer Vitalograph® (Ennis, Ireland). Instructions for the maneuvers were made following the recommendations of the Brazilian Pneumology and Tisiology Society for pulmonary function testing.Citation10 FVC values were then compared with expected values for the Brazilian population, adjusted for gender and age.Citation12
Six-minute walk test
The 6MWT was performed according to the American Thoracic Society protocolCitation13 prior to the HD session. The distance walked (in m) was recorded and the following measurements were made before and at the end of the test: BP, respiratory rate (RR), subjective effort perception according to the Borg scale from 6 to 20,Citation14 heart rate (HR), and peripheral oxygen saturation (SpO2) using a pulse oximeter (Handheld Pulse Oximeter Model 512, Respironics®, Andover, MA, USA). In order to estimate the optimal distance to be walked, walked distance prediction formulas were used according to gender, as proposed by Enright and Sherrill.Citation15 Test interruption criteria were the following: SpO2 < 87%, development of dizziness, headache, vomiting, chest pain, dyspnea, fatigue, and/or cramps.
QoL questionnaire
The KDQOL-SF contains some items of the generic SF-36 Questionnaire (Medical Outcomes Study 36) and a part specifically dedicated to kidney disease. This instrument was validated for the Brazilian population by Duarte et al.Citation16 Scores for every dimension range from 0 to 100, with higher scores reflecting a better QoL.Citation17
Laboratory measurements
Blood was collected to measure hematocrit and hemoglobin, and serum levels of urea, creatinine, potassium, phosphorus, high sensitivity C-reactive protein (hsCRP), and albumin. Blood was collected twice, at baseline and on the 70th day of the program, before the HD session. Efficiency of HD was measured by Kt/V, using the second generation formula of Daugirdas.Citation18
Training program
The training program had a length of 10 weeks with every patient performing 30 training sessions for the respiratory muscles or peripheral muscles in the first 2 h of HD in the sitting position.
The respiratory training program of the RMT group consisted of training the inspiratory muscles, while the peripheral muscle program of the PMT group trained the knee extensor muscles. The parameters number of sets, number of repetitions, rest pauses between sets, and percentage of one maximum repetition test (1RM) and PImax established for the RMT and PMT groups met the criteria for strength training.Citation19
The RMT group underwent training during HD using the Threshold Loader® (Philips Respironics, Amsterdam, The Netherlands) using 50% of PImax. On the 30th day, the respiratory pressure was measured to adjust the load of the Threshold Loaded® Inspiratory Muscle Trainer (IMT) during the program. The Threshold Loaded® IMT has a unidirectional flow-independent valve to ensure constant resistance (linear load) and enable a specific pressure adjustment. The exercise load was changed throughout the training according to 50% of PImax found at 30 days. Patients performed three sets of 15 inspirations at the equipment mouthpiece and rested for 60 seconds.
The PMT group performed training during HD using free leg weights at the distal extremity of their lower limbs. The load for every patient was set according to the 1MR.Citation19 The training load was 50% of this 1MR and was changed according to the reassessment performed on the 30th day. Patients performed a total of three sets of 15 knee extension repetitions, resting for 60 s in between.
The control group underwent the same assessments as the other groups, at the baseline and on the 70th day, without any intervention.
Statistical Analysis
The descriptive statistics are presented as percentages for qualitative data and as mean (SD) or median and interquartile ranges for quantitative data. The Shapiro–Wilk test was used to assess the normality of continuous variables. Chi-square test and analysis of variance (ANOVA) were used to compare the three groups, and Kruskal–Wallis test was used for variables with asymmetric distribution. A post-hoc analysis was made with the Bonferroni correction for the multiple comparisons.
Analysis of Covariances (ANCOVA) was used to assess variation [Δ = estimated mean (standard error) of the covariance analysis of delta values corrected by baseline values] of the functional and laboratory variables in the three groups, corrected for baseline values. The results were presented as mean (standard error). Correlations between functional variables were assessed by Pearson’s coefficient or Spearman’s coefficient in case of an asymmetric distribution. The size of the difference of the intervention effect among the groups was assessed using the “standardized effect size” calculation with a confidence interval (CI) of 95%. Values greater than 0.50 were considered as having an effect size of greater magnitude.Citation20
Data were processed and analyzed using the Statistical Package for Social Sciences (SPSS, Inc., Chicago, IL, USA) for Windows, version 18.0. The significance level was established at p < 0.05.
RESULTS
Of the 45 patients initially included, six did not complete the study protocol due to non-compliance (n = 5) or death (n = 1) and were not included in the analysis. Therefore, 39 patients were studied, with an average age of 48.3 (12.1) years (ranging from 19 to 69 years); 23 (59%) were males. Hypertension was most prevalent (35.9%) as etiology of CKD, followed by diabetes mellitus (15.4%). The number of comorbidities and the median time on HD did not differ among the three groups, as shown in . Weight variation in the HD session, which corresponds to the actual ultrafiltration, did not show any statistical difference among the three groups either at the baseline () or at 70 days. Baseline Kt/Vsp in RMT and C groups was 1.5 (0.3), and in the PMT group was 1.4 (0.2) (p = 0.85), which is compatible with an adequate dialysis dose. The urea reduction ratio (URR, %) was also similar among the groups at baseline, with the following values: RMT = 69.9 (8.8), PMT = 67.5 (9.4), and C = 72.2 (6.8) (p = 0.34).
Table 1. Demographic and clinical data of the patients on HD.
Functional variables at baseline and the variation of these parameters after training in relation to respiratory pressure measurements, spirometry, and the 6MWT are shown in . Mean PImax, PEmax, FVC, and 6MWT results did not differ among the three groups at baseline. In the post-intervention measurement, both absolute PImax and the percentage PImax in relation to the predicted value were significantly increased in the RMT group, and this increase was higher than in the PMT group (Δ% of predicted, p = 0.023) and C group (Δ% of predicted, p < 0.001). The positive variation in PMT group was also significantly higher as compared to the C group, but this increase was of a lesser magnitude (Δ% of predicted value, p = 0.008).
Table 2. Respiratory and peripheral functional variables before and after the muscle training.
The proportion of patients that achieved the predicted value for baseline PImax, adjusted for age and gender, were 60.6% (RMT), 55.1% (PMT), and 73.5% (C) (p = 0.12). At baseline, only three subjects, one from each group, achieved the predicted PImax, while post training, eight patients achieved the predicted, being four patients in RMT group, three in PMT group, and one in the C group. Regarding the post-training measure, the RMT group achieved 82.3% of the predicted value, whereas the PMT and C groups achieved 64.7% and 67.6%, respectively. However, this difference was not statistically significant, which is probably due to the small sample size (p = 0.18).
A statistically significant difference was found between the RMT and C groups for both absolute PEmax (p = 0.016) and the percentage PEmax in relation to the predicted value post training (p = 0.049), as shown in . At baseline, RMT, PMT, and C groups achieved 67.5%, 69.4%, and 86.7% of the predicted PEmax, respectively (p = 0.10). However, seven patients exceeded the predicted value, both in the baseline and in the post-intervention assessment.
No statistical difference was found in the achieved FVC in relation to the predicted value both at baseline (p = 0.44) and post intervention (p = 0.97). However, after the training, six patients achieved 100% or more of the predicted value (three in the RMT group, one in the PMT group, and two in the C group) and in the baseline assessment only three subjects achieved this score (one in the RMT group and the same two patients in the C group).
Predicted distances to walk in the 6MWT at baseline for RMT, PMT, and C groups were 635.6 (72.9) m, 601.6 (69.6) m, and 565.1 (65.7) m, respectively. The values achieved after the intervention were 519.0 (103.8) m, 475.1 (74.1) m, and 407.0 (116.7) m, respectively (p = 0.026). There was a significant increase in the distance walked (positive variation), both for absolute and the percentage of predicted values, when comparing RMT group (Δ% of predicted value, p < 0.001) and PMT group (Δ% of predicted value, p = 0.019) to controls. The positive variation shown by the RMT group was of a greater magnitude than that found for the PMT group.
Correlation analysis of pulmonary function, respiratory muscle strength (PImax and PEmax), and distance walked at the 6MWT showed significant results. A positive and significant correlation was found between variation of the distance walked in the 6MWT and variation of both PImax (r = 0.508, p = 0.001) and PEmax (r = 0.457, p = 0.003). Although of borderline significance, there was also a correlation between distance walked in the 6MWT and FVC (r = 0.313, p = 0.05).
Biochemical and inflammatory profile of the patients at baseline was similar in the three groups (), except for the lower serum creatinine levels found in controls. There was a significant increase in hematocrit values, hemoglobin, and serum albumin levels in the training groups, with the most significant difference found in the RMT group. Likewise, the RMT and PMT groups showed a reduction in serum potassium and phosphorus levels after 70 days of muscle training, with a negative variation in relation to the C group (). No significant variation of Kt/V at the end of the training was found in RMT, PMT, and C groups 0.1 (0.07), 0.08 (0.1), and 0.1 (0.1), respectively (p = 0.93).
Table 3. Biochemical and inflammatory parameters before and after muscle training of patients on HD.
CRP had an asymmetric distribution and was analyzed by the Kruskal–Wallis test. Baseline CRP levels were similar in the three groups (p = 0.42). After 10 weeks of muscle training, there was a significant reduction in CRP levels in the RMT group [−6.1(−18.7 to −0.4)] and in the PMT group [−2.2(−8.6 to −0.4)] when compared to controls [0.7(−0.7–6.6) vs. RMT, p = 0.024 and vs. PMT, p = 0.026] ().
Figure 1 . Median of C-reactive protein (mg/L) at baseline and after ten weeks of muscle training in respiratory and peripheral training and in control groups (Δ RMT vs. Δ C, p = 0.001; Δ PMT vs. Δ C, p = 0.03).
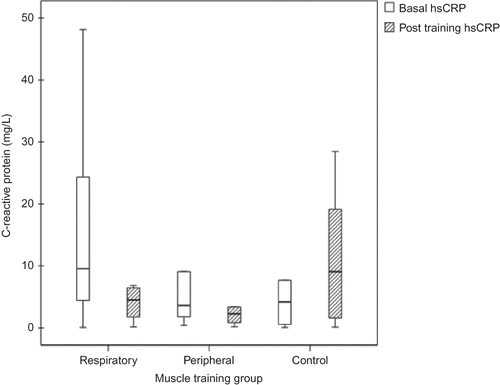
Figure 2 . Improvement in quality of life domains after ten weeks of muscle training in respiratory and peripheral training and in control groups.
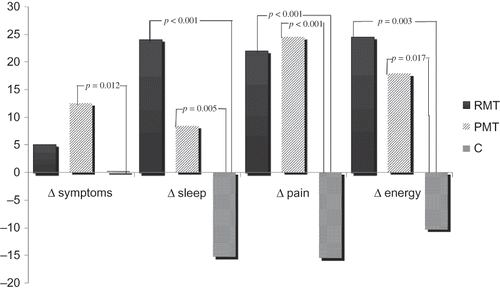
Regarding the QoL parameters, the training groups showed a significant increase in four scores that did not occur in controls. This was observed specifically for the following domains: energy/fatigue (p = 0.002), sleep (p < 0.001), pain (p < 0.001), and list of symptoms/problems (p = 0.014). The latter score, however, was similar in RMT and C groups (p = 0.32). Functional capacity and physical aspects post-training scores were similar.
Variation of QoL domains after training is shown in . In order to measure the magnitude of the effect of muscle training on functional, biochemical, and QoL parameters, effect size was calculated on these variables. The magnitude of the effect size was systematically greater in the RMT group, but a significant effect size was also found in the PMT group for all functional variables assessed. Analysis of the variation of hematocrit, hemoglobin, creatinine, albumin, potassium, and phosphorus levels showed the same trend; that is, the effect size was greater in the RMT than in the PMT group (). Effect size on urea clearance in the HD session was small in both groups. In relation to the effect size on the QoL parameters—energy/fatigue, sleep, and pain—a similar pattern was found, with a more significant effect in the RMT group than in the PMT group. However, in the item list of symptoms/problems, effect size was significant only in the PMT group (0.95 vs. 0.47 in the RMT group).
Table 4. Effect size of muscle training on functional and biochemical variables.
DISCUSSION
Physical training exercise has been recognized as an adjunctive therapy that can improve physical function and perhaps influence the efficiency of HD in patients with ESRD.Citation21–24 However, it has not been routinely prescribed as a treatment modality for this patient population, unlike the practice routinely used in the management of heart and pulmonary diseases.Citation25,26 Presently, there are no clinical guidelines establishing a reference protocol for dialysis patients. The use of different methods, such as aerobic protocols used either alone or in combination with muscle strength training, shows the lack of consensus on which training is most efficient.
Patients with ESRD have decreased strength and endurance of their inspiratory muscles in the basal state.Citation27 Our study also found a decrease in baseline inspiratory and expiratory muscle strength in the order of 37% and 25%, respectively, compared to reference standards. This finding corroborates other studyCitation28 that reported a 30% to 40% reduction in the inspiratory muscle strength in patients with ESRD, when compared to healthy subjects. The pathophysiological basis of these changes includes several factors, such as muscle atrophy of type I and type II fibersand impairment in oxygen transport, uptake, and consumption.Citation29,30 Additionally, complications of the uremic syndrome directly affect the functional capacity and QoL of these patients, as anemia, neuropathy, muscle wasting, bone mineral disorders, and vascular calcifications are common findings in ESRD.Citation28
Reduction in functional capacity is one of the main complaints of patients on dialysis, manifested by decreased tolerance to exercise and to daily life activities. Both uremic myopathy and HD procedure themselves promote protein breakdown,Citation31 affecting peripheral and proximal muscles with a strong impact on the overall physical performance. After 10 weeks of inspiratory muscle strength training, we found a significant improvement in PImax with a mean positive variation of 15% of achieved value in relation to the predicted. Moreover, a correlation was found between PImax and the distance walked in the 6MWT, suggesting that strength training of inspiratory muscles can improve the functional performance. Although no expiratory muscle training was performed, there was also a significant increase in PEmax, which could be related to the work imposed onto the abdominal muscles during the intervention, as suggested by Coelho et al.Citation32
The training modality most widely used in clinical trials for patients on HD is aerobic exercise using a cycle ergometer for approximately 30 minutes or for as long as the patient can tolerate.Citation33 More specific muscle strength training methods have been less studied and there are many different protocols in this regard. These protocols basically constitute low to moderate intensity resistance exercisesCitation1,7,21,34 for the upper limbs,Citation22–24 lower limbs,Citation21,22,24 and abdominal muscles.Citation22–24 Few training protocols involve the prescription of progressive resistance exercises.Citation23 As far as we know, there are no studies following this type of protocol, where duration of exercise is not previously established, but the number of sets and repetitions is strictly controlled. Muscle strength training with previously established load and number of sets and repetitions was chosen because it is easy and rapid to perform.
The 6MWT is a safe procedure to assess functional capacity which is reduced in ESRD patients.Citation5,7 This finding was confirmed in our study where 97% of the patients had a significant reduction in the distance walked at baseline in relation to the predicted value. After muscle training, however, there was a significant increase in the distance walked, with a more significant positive variation found in the group who underwent respiratory training. After 12 weeks of strength training, Headley et al.Citation34 also reported an increase in the distance walked at the 6MWT in patients with ESRD on HD.
These findings give support to the idea that functional capacity is influenced by cardiorespiratory conditioning and not only by peripheral factors such as muscle weakness, neuropathy, and myopathy.Citation1 Our study showed a positive correlation between PImax and the 6MWT, with both training methods positively changing the functional performance of the patients. These data suggest that impairment of the functional capacity can be attenuated by a strength gain, both in peripheral and respiratory muscles.
It is still controversial if muscle training can improve HD efficiency.Citation4,5,35 The absence of an effect of the exercise training on Kt/V may be related to the short duration of the program, perhaps because a “muscle morphologic threshold” may be necessary to increase muscle blood flow and capillary surface area with solute transfer to the intravascular compartment.Citation4,5 We did not find any effect of the exercise training on Kt/V, but we found a significant decrease in the levels of serum phosphorus, creatinine, and potassium. Considering that our patients were clinically and metabolically stable, we could speculate that the exercise led to an improvement in these biochemical parameters. Other authorsCitation36,37 reported higher levels of urea and potassium clearance in the group who underwent training. If this could be related to increased availability of these molecules to central circulation allowing more effective clearance is still unproven.Citation38
Studies that performed aerobic training during dialysis have shown an increase in hemoglobin and hematocrit after the program.Citation33 This seems to be an unexpected finding, but we could not input this higher hematocrit to hemoconcentration resulting from a higher ultrafiltration rate, because weight reduction at the end of the dialysis session did not differ from controls. Erythropoietin dose does not explain these differences either, because training groups received a dose similar to that given to controls. Alternatively, one explanation could be the decrease in inflammatory status as evidenced by a reduction in hsCRP levels () lowering the resistance to erythropoietin action. The etiology of systemic inflammation in CKD is complex and multifactorial and has not been fully elucidated yet. Kaizu et al.Citation39 have shown an inverse relationship between muscle mass and serum level of IL-6 and CRP in patients with ESRD. Cross-sectional studies have also pointed to an inverse relationship between systemic inflammation and physical activity.Citation40 Kopple et alCitation.41 compared the use of endurance versus strength training, independently or in combination, reporting no improvement in serum IL-6 and hsCRP in the training groups. However, in other studyCitation42 physical exercise during HD attenuated the chronic inflammatory state of uremia. Cheema et al.Citation23 investigated the impact of strength training with progressive load for 12 weeks in patients with ESRD and also found a significant reduction in CRP levels. In our study, we found a decrease in CRP levels from baseline to the 10th week in the muscle training groups, which was more prominent in the RMT group. In controls, median of CRP did not change. This finding, together with a slightly increased serum albumin, could indicate a positive impact of muscle training on the nutritional and inflammatory status of these patients. Another issue to consider is that improvement of the sleep pattern, as we found in this study, could be related to the reduction in hsCRP levels, as suggested by Chen et al.Citation42 Other unmeasured confusing factors still have to be considered, for instance, the engagement in a training program with a different management of health and self care, resulting in more compliance and adherence to dialysis team prescription.
Some studies that have investigated the influence of physical exercise on QoL reported benefits in several domains.Citation7,8,21,24 Higher scores of physical function and physical capacity were achieved after 16 weeks of muscle training, half of the time at home and half of the time during HD.Citation7 Improvements in mental and physical components have also been described after 3 months of strength training in the lower limbs during dialysis, in combination with 30 minutes of exercise in a cycle ergometer.Citation21 In this study, we found higher scores in the pain, fatigue, sleep, and problems/symptoms domains in a self-assessment made by the patients after 10 weeks of muscle training. It seems logical to assume that there is a connection between physical conditioning and a greater tolerance to daily activities.
In conclusion, this randomized controlled trial showed that respiratory and peripheral muscle training during dialysis for 10-week improved functional performance of patients with ESRD. Although an improvement was also found in biochemical and inflammatory markers, a direct cause and effect relationship could not be determined because other factors that influence the metabolic condition of these patients were not assessed. If a strength training program administered for a longer period improves HD efficiency remains to be determined.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. All authors participated in the approval of the final version of the manuscript. They also certify that neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere.
Financial support: Research Funding of Hospital de Clínicas de Porto Alegre (FIPE/HCPA).
REFERENCES
- Kouidi E, Albani M, Natsis K, . The effects of exercise training on muscle atrophy in hemodialysis patients. Nephrol Dial Transplant. 1998;13:685–699.
- Bianchi P, Menna Barreto SS, Thomé FS, Klein AB. Repercussion of hemodialysis on the pulmonary function of terminal chronic renal patients. Braz J Nephrol. 2009;3:25–31.
- Cheema BS, Singh MA. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol. 2005;25:352–364.
- Parsons TL, Toffelmire EB, King-VanVlack CE. The effect of an exercise program during hemodialysis on dialysis, efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. Clin Nephrol. 2004;61:261–274.
- Parsons TL, Toffelmire EB, King-Van Vlack CE. Exercise training during hemodialysis improves dialysis efficacy and physical performance. Arch Phys Med Rehabil. 2006;87:680–687.
- Cheema BS, Abas H, Smith BC, . Effect of resistance training during hemodialysis on circulating cytokines: a randomized controlled trial. Eur J Appl Physiol. 2011;111:1437–1445.
- Painter P, Carlson L, Carey S, Paul SM, Myll J. Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. Am J Kidney Dis. 2000;35:482–492.
- Painter P, Moore G, Carlson L, . Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis. 2002;39:257–265.
- Segura-Ortí E. Exercise in hemodyalisis patients: a literature systematic review. Nephrology. 2010;30:236–246.
- Souza RB. Brazilian Society of Pneumology and Tisiology: Guidelines for pulmonary function tests. Maximal static respiratory pressures. J Bras Pneumol. 2002;28(Suppl. 3):S155–S165.
- Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests: II: maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32:719–727.
- Pereira CA, Barreto SP, Simões JG, Pereira FW, Gerstler JG, Nakatami J. Reference values for spirometry in Brazilian adults. J Bras Pneumol. 1992;18:10–22.
- ATS. ATS statement: guidelines for the six-minute walk-test. Am J Respir Crit Care Med. 2002;166:111–117.
- Borg G, Noble B. Perceived exertion. In: Wilmore J, ed. Exercise and Sport Sciences Reviews. Vol. 2. New York: Academic Press; 1974:131–153.
- Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387.
- Duarte PS, Ciconelli RM, Sesso R. Cultural adaptation and validation of the “Kidney Disease and Quality of Life–Short Form (KDQOL-SF 1.3)” in Brazil. Braz J Med Biol Res. 2005;38:261–270.
- Korevaar JC, Merkus MP, Jansen MA, Dekker FW, Boeschoten EW, Krediet RT. Validation of the KDQOL-SF: a dialysis-targeted health measure. Qual Life Res. 2002;11:437–447.
- Daugirdas J, Schneditz D. Postdialysis urea rebound: measurement, prediction and effects of regional blood flow. Dial Transplant. 1994;23:166–173.
- Shimano T, Kraemer WJ, Spiering BA, . Relationship between the number of repetitions and selected percentages of one repetition maximum in free weight exercises in trained and untrained men. J Strength Cond Res. 2006;20:819–823.
- Hopkins WGA. New View of Statistics. 2005. Available at: http://www.sportsci.org/resource/stats/index.html. Accessed June, 2011.
- Oh-Park M, Fast A, Gopal S, . Exercise for the dialyzed: aerobic and strength training during hemodialysis. Am J Phys Med Rehabil. 2002;81:814–821.
- Cheema BS, O’Sullivan AJ, Chan M, . Progressive resistance training during hemodialysis: rationale and method of a randomized-controlled trial. Hemodial Int. 2006;10:303–310.
- Cheema B, Abas H, Smith B, . Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–1601.
- Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol. 2006;17:2307–2314.
- Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest. 2003;124: 1357–1364.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol. 1999;22:727–732.
- Weiner P, Zidan F, Zonder HB. Hemodialysis treatment may improve inspiratory muscle strength and endurance. Isr J Med Sci. 1997;33:134–138.
- Bohannon RW, Hull D, Palmeri D. Muscle strength impairments and gait performance deficits in kidney transplantation candidates. Am J Kidney Dis. 1994;24:480–485.
- Prezant DJ. Effect of uremia and its treatment on pulmonary function. Lung. 1990;168:1–14.
- McIntyre CW, Selby NM, Sigrist M, Pearce LE, Mercer TH, Naish PF. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant. 2006;21:2210–2216.
- Ikizler TA, Pupim LB, Brouillette JR, et al. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol Endocrinol Metab. 2002;282:107–116.
- Coelho DM, Castro AM, Tavares HA, . Effects of a physical exercising program on conditioning of hemodialysis patients. Braz J Nephrol. 2006;28:121–127.
- Goldberg AP, Geltman EM, Hagberg JM, . Therapeutic benefits of exercise training for hemodialysis patients. Kidney Int (Suppl). 1983;16:303–309.
- Headley S, Germain M, Mailloux P, . Resistance training improves strength and functional measures in patients with end-stage renal disease. Am J Kidney Dis. 2002;40:355–364.
- Załuska A, Załuska WT, Bednarek-Skublewska A, Ksiazek A. Nutrition and hydration status improve with exercise training using stationary cycling during hemodialysis (HD) in patients with end-stage renal disease (ESRD). Ann Univ Mariae Curie Sklodowska Med. 2002;57:342–346.
- Vaithilingam I, Polkinghorne KR, Atkins RC, Kerr PG. Time and exercise improve phosphate removal in hemodialysis patients. Am J Kidney Dis. 2004;43:85–89.
- Kong CH, Tattersall JE, Greenwood RN, Farrington K. The effect of exercise during hemodialysis on solute removal. Nephrol Dial Transplant. 1999;14:2927–2931.
- Schneditz D, Daugirdas JT. Compartment effects in hemodialysis. Semin Dial. 2001;14:271–277.
- Kaizu Y, Ohkawa S, Odamaki M, . Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis. 2003;42:295–302.
- Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–1876.
- Kopple JD, Wang H, Casaburi R, . Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol. 2007;18:2975–2986.
- Chen HY, Cheng IC, Pan YJ, . Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney Int. 2011;80:415–422.