Abstract
Purpose: The choice of vascular access catheter may affect filter life during continuous renal replacement therapy (CRRT). In particular, differences in catheter design might affect the incidence of circuit clotting related to catheter malfunction. Design and setting: Sequential controlled study in a tertiary, adult intensive care unit (ICU). Aim: To compare circuit life when CRRT was performed with a Niagara™ catheter or a Medcomp™ catheter. Patients and measurements: We studied 46 patients with acute kidney injury requiring CRRT, all delivered with catheters in the femoral position. We obtained information on age, gender, disease severity score [acute physiology and chronic health evaluation (APACHE II) and APACHE III], filter life, heparin dose per hour, daily systemic hemoglobin concentration, platelet count, international normalized ratio (INR), and activated partial thromboplastin time (APTT) during CRRT. Results: We studied 254 circuits in 46 patients. Of these, 26 patients (140 circuits) used the Niagara catheter and 20 patients (114 circuits) used the Medcomp catheter. Median circuit life in the two groups were 11 h and 7.3 h, respectively (p < 0.01). Patients using Medcomp catheters had a lower platelet count (p = 0.04) and a lower hemoglobin concentration (p = 0.01), but INR (p = 0.16), APTT (p = 0.46), anticoagulant treatment (p = 0.89), and heparin dose per hour (p = 0.24) were similar. After correcting for confounding variables by multivariable linear regression analysis, it was found that the choice of catheter is not an independent predictor of circuit life. On Kaplan–Meier survival analysis, circuit life was not significantly different between the two catheters (p = 0.87). Conclusion: The choice of either the Niagara or Medcomp catheter does not appear to be a significant independent determinant of circuit life during CRRT.
INTRODUCTION
Continuous renal replacement therapy (CRRT) is commonly used in critically ill patients with acute kidney injury.Citation1–3 During CRRT, catheter-related problems are a matter of substantial concern.Citation4 Adequate vascular access is, therefore, a key prerequisite for successful treatment delivery. In addition, microthrombus formation in the catheter remains a major problem that compromises dialysis adequacy, shortens access lifespan, and results in circuit failure.Citation4–9
Different catheters are available in the market for CRRT including bismuth-coated cathetersCitation10–12 and catheters of different lumen design, diameter, and cross-sectional surface. These properties may influence circuit life and therefore have practical implications. In the clinical setting, there are varied dual lumen vascular access catheters currently available for patients requiring CRRT.Citation4 The type and design of the catheter may influence pressure gradients across the catheter and resistance to blood flow through the two lumens. The properties that are likely to influence the resistance of the catheter include its internal diameter, length, the shape of blood entry holes, and their location.Citation5 Two widely used CRRT catheters, which have a different design, are the Niagara™ and Medcomp™ catheters. Although these catheters share a similar French (Fr ) size, they differ in structure, properties, and geometry. It is possible that these differences in design affect catheter function, resistance to flow, flow fluctuations, and ultimately result in different circuit life when applied in continuous use for intensive care unit (ICU) patients, where many nursing-related patient movements and procedures affect the flow through these catheters. Accordingly, we conducted a pilot study to investigate whether choice of one of these two catheters for CRRT in critically ill patients was independently associated with a different circuit life.
METHODS
We performed a prospective before-and-after observational study of the impact of choice of double lumen central vascular access catheter on circuit life. The study was approved by the local Institutional Review Board, which waived the need for informed consent.
We compared circuit life using two different catheters: the Niagara 13.5-Fr catheter (24 cm long, uneven double D shape, and elliptic and angled arterial inflow opening, internal diameter of 2.18-mm (for both lumens) catheter (Bard, Murray Hill, NJ, USA) and the Medcomp Hemocath, S/DL139E 13.5-Fr 24-cm silicon catheter (Medcomp, Harleysville, PA, USA). This catheter has a double lumen internal design, where the internal design is configured as a lumen within a lumen or is considered a coaxial configuration. The arterial lumen has a cross-sectional area of 2.64 mm2 and an equivalent internal diameter of 1.83 mm and includes additional side wall holes to draw in blood; the venous lumen has a cross-sectional area of 3.85 mm2 and an equivalent diameter of 2.21 mm. A schematic diagram of both end and side profiles for these catheters is provided in . We refer to these catheters throughout the article as the Niagara and Medcomp catheters.
Figure 1. Diagram illustrating the differences in the characteristics and design of the Niagara and the Medcomp catheters.
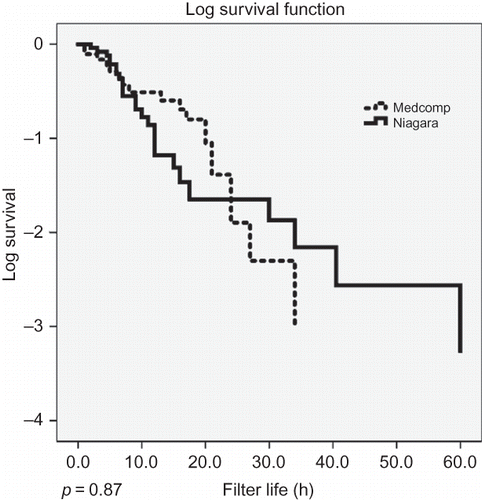
Figure 2. Kaplan–Meier product limit survival plots of circuit life with the Niagara and the Medcomp catheters.
We first used Niagara catheters exclusively and obtained data of 26 consecutive patients (before period). We then switched to exclusive use of the Medcomp catheters and applied them to 20 consecutive patients (after period). To remove the confounding effect of different vascular access site, we only studied patients who received CRRT via the femoral vein, which is the preferred approach in our ICU.
The study was limited exclusively to patients undergoing CRRT as continuous venovenous hemofiltration (CVVH). Patients receiving CVVH or intermittent dialysis were not included in the study.
We used AN69ST (ST 100) 1.0 M2 (Gambro Nephral TM, Lund, Sweden) or DF 140 Polyethersulfone 1.4 M2 (Infomed Polyethersulfone, Geneva, Switzerland) hollow-fiber filters for all treatments. We set the blood flow (Qb) at 200 mL/min for all circuits. We used bicarbonate-buffered fluid (Hemosol BO, Gambro, Sondalo, Italy or Accusol, Baxter, Sydney, Australia). Replacement fluid was delivered into the extracorporeal circuit before and after the filter (i.e., pre- and postdilution), with a ratio of 50% predilution and 50% postdilution replacement fluid. The effluent was prescribed at a standardized rate of 2 L/h. Anticoagulation was conducted according to a predefined ICU protocol. This anticoagulation protocol prescribed no anticoagulation in the immediate period after major surgery (cardiac surgery, aortic surgery, and neurosurgery) and in patients who were at risk of bleeding (coagulopathy, thrombocytopenia, recent intracranial hemorrhage, or trauma). In other patients, prefilter heparin at 5–10 IU/kg/h is prescribed.
For the purpose of the study, circuit clotting or failure was defined as one or more of the following: a transmembrane pressure (TMP) greater than 250 mmHg, a prefilter pressure greater than 200 mmHg, extracorporeal circuit clot obstructing blood flow, or any alarm device that signals the filter has clotted. Circuits were not included in the study if they were electively removed for transport [e.g., computerized tomography (CT) scans, transfer to the operating theater], or elective discontinuation of therapy.
Demographic and clinical data [age, gender, diagnosis, and acute physiology and chronic health evaluation (APACHE) score] were obtained at ICU admission. The following data were also obtained from our prospectively recorded circuit life monitoring system: circuit life (h), anticoagulation type and dose, and then daily (0500 h) hemoglobin (g/dL), platelet count, international normalized ratio (INR), and activated partial thromboplastin time (APTT) (second).
Statistics
Study variables were not normally distributed and were reported as medians with inter-quartile range (IQR). Continuous variables were compared using the Mann–Whitney U-test. Comparison of nominal data was performed using Fisher’s exact test. Circuit life for the two groups was presented graphically using Kaplan–Meier product limit survival plots. The log-rank test was used to compare circuit life between the two groups. We used multivariable linear regression analysis to relate catheter type to the duration of circuit life after correcting for confounding variables. Given a mean filter life of 15 h in one group and a standard deviation of 11 h, this study had an 80% power of detecting a 3-h difference in mean filter life at an alpha of 0.05. A p-value ≤0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
We analyzed 254 circuits in 46 patients receiving CRRT via femoral vein vascular access. Among these patients, 26 (140 circuits) received CRRT with Niagara catheter and 20 (114 circuits) received CRRT with the Medcomp catheter.
The patients’ demographic profiles, coagulation treatment, and median circuit life are shown in . In the Niagara group, 5 patients had acute chronic liver failure, 8 had septic shock, and 6 had fulminant liver failure; 4 received CRRT after cardiac surgery, 2 during cardiogenic shock, and 2 after hemorrhagic shock; 1 after overdose, 1 after cardiac arrest, and 1 after rhabdomyolysis. In the Medcomp group, 6 patients had acute chronic liver failure, 5 had septic shock, and 2 had fulminant liver failure; 2 required CRRT after cardiac surgery and 2 after cardiac arrest; 1 after liver transplantation, 1 after massive pulmonary embolism, and 1 during cardiogenic shock. Sixteen (80%) patients in the Medcomp group received mechanical ventilation compared with 20 (76.9%) patients in the Niagara group.
Table 1. Comparison of Niagara catheter with Medcomp catheter.
Two hundred and fifty-four circuits were observed. Among these circuits, 170 (67%) were treated with no anticoagulation. Median circuit life with the Niagara catheter was 11 (IQR: 7–19) h compared with 7.3 (IQR: 4.5–15.3) h (p < 0.01) with the Medcomp catheter [mean values: 14.9 (±11) h vs. 10.8 (±8) h]. In addition, the characteristics of filters above or below 10 h of function are presented in .
Table 2. Comparing the characteristics of filters (filters that had a life >10 h versus filters that had a life <10 h).
Table 3. Multivariable linear regression analysis of factors potentially associated with circuit life.
Hourly heparin dose delivered during CRRT (p = 0.24), INR (p = 0.16), and APTT (p = 0.46) were not significantly different between the two groups, but the group using the Medcomp catheter had a lower platelet count (p = 0.04) and a lower hemoglobin concentration (p < 0.01). Kaplan–Meier survival plots of circuit life are shown in . The log-rank test comparison between the two groups found no difference in filter life (p = 0.870).
On multivariable linear regression analysis, the mean value of circuit life was significantly correlated (r2 = 0.26) with APTTs (p < 0.01) but not with catheter type ().
DISCUSSION
Key Findings
We compared the impact of two vascular access catheters of different design on circuit life during CRRT in 46 patients and 254 circuits, all treated with femoral vein access. We found that, although median circuit life was 11 h using the Niagara catheter compared with 7.3 using the Medcomp catheter, there was no significant difference in circuit survival using log-rank assessment. Furthermore, after correcting for confounding variables by multivariable linear regression analysis, choice of catheter was not a predictor of circuit life, but APTT was.
Comparison with Previous Studies
Few studies have assessed the clinical impact of using different catheters during CRRT. In a recent study, Meier et al.Citation12 found that, compared to an identical noncoated catheter, a bismuth-coated catheter was associated with a lower incidence of catheter dysfunction, catheter thrombosis, and catheter-related bacteremia. However, these investigators studied catheters inserted in either the jugular or the subclavian vein and excluded patients who received CRRT via the femoral vein. They also reported the outcome of catheter dysfunction but did not comment on the effect of choice of catheter on circuit life. Patient selection in the study by Meier et al. meant that only catheters of 20 cm in length were used and that the findings may, therefore, not apply to patients receiving CRRT via the femoral vein using a 24-cm catheter. This lack of information is unfortunate, because femoral catheters are frequently used in the delivery of CRRTCitation12 and because such catheters are longer (24 cm) and have unique risks and functional properties. In particular, they have a lower rate of colonization compared with the jugular position in patients with a lower body mass index (BMI).Citation13 In addition, such catheters may have a slightly lower incidence of dysfunction.Citation12 Finally, although previous comparisons have been made between bismuth-coated catheters and an equivalent noncoated catheter, such comparisons may not be relevant to units that routinely use a bigger catheter, such as the Niagara, which is 13.5 Fr in size and, thus, statistically less likely to develop dysfunction.
Previous studies have addressed the potential impact of catheter size on ease of blood flow maintenance by measuring their resistance to flow ex vivo.Citation14,15 Such studies have shown that large gauge (>13 Fr) catheters pose significantly lower resistance to flow than small gauge catheters [13] and that, among long catheters (>19 cm), the Niagara catheter posed the lowest resistance to flow.Citation14 Such catheters are typically used for femoral access.
For the above reasons, we compared the Medcomp catheter with the Niagara catheter in terms of their impact on circuit life, a major concern for critical-care physicians. Our findings support the notion that when 13.5 Fr catheters of different designs are compared, no major differences are detected.
To our knowledge, there have not been any direct in vivo comparisons of two larger catheters such as the Niagara and Medcomp both of a gauge, which appears to be the best ex vivo. The other studies only identifiable by electronic reference libraries which have assessed catheter function have also been ex vivoCitation16 or in pigs.Citation17 Their applicability to humans remains uncertain.
Strengths and Limitations
Our study is the first to compare the impact on circuit life of two 13.5-Fr-sized, 24-cm-long CRRT catheters inserted in the femoral vein. It is also the first to compare a very commonly used catheter in Australia, the Niagara, with a similarly sized catheter of a different design widely used in the United States, the Medcomp. Thus, the study findings are highly relevant to all units that prefer larger catheters and the femoral position. However, this is a single-center study and its findings may not apply to centers that prefer to use catheter insertion at other sites. We note that randomized controlled trialsCitation18 show no difference in catheter dysfunction rate between jugular or femoral access. These observations support the view that the use of the femoral vein for CRRT is rational and justified, perhaps with the possible exception of obese patients. We did not systematically assess catheter function by measuring flows and negative applied pressures. However, although these measurements are of some importance, the most important aspect of catheter function relates to whether it affects circuit life. We found no difference between the two catheters. Because we only studied 46 patients and 254 circuits, we cannot comment on the risk of catheter colonization to catheter-related bacteremia. We note, however, that in our unit, in 2009, we exclusively used Niagara catheters for a total of >600 patient days and did not record a single episode of catheter-related bacteremia (Source data: Victorian Nosocomial Infection Survey System—ICU line surveillance program). We report a short filter life. However, we are a liver transplantation referral center. Thus, close to 50% of our CRRT patients have liver disease with high risk of both bleeding and clotting simultaneously and are unable to receive citrateCitation19 due to the liver’s failure to metabolize it. It is for these reasons that two-thirds of our patients received CRRT without anticoagulation. Unfortunately, such patients’ procoagulant stateCitation20 makes filter clotting frequent. In similar liver patients and similar circumstances, other investigators have reported similarly short mean filter life.Citation21
We did not collect information on the number of catheter changes because of dysfunction. However, such events are relatively rare in our unit (<10%). In addition, we do not have information on the duration of catheter placement. However, the mean duration of CRRT in our unit is in keeping with the findings of the randomized evaluation of normal versus augmented level of dialysis (RENAL) trial, of which it was a leading site, averaging 6 days per patient.Citation22 Given such relatively short duration of treatment, as mentioned above, catheter changes are infrequent. In addition, blood products such as fresh frozen plasma and/or platelets can accelerate filter clotting. We did not record any data for administration of these blood components; however, we could only assume that over the study period, these may have equal influence on each group or period of time each catheter was in use. Finally, the differences in cost between the two catheters ($147 for the Niagara catheter and $125 for the Medcomp catheter) are small.
CONCLUSION
After adjustment for confounding variables, the Niagara catheter and the Medcomp catheter appear to deliver similar circuit life when used in the femoral position during CRRT. Until further information is available, the choice of one catheter over the other should be based on clinician preference and local availability.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Uchino S, Bellomo R, Morimatsu H, . Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33:1563–1570.
- Vanholder R, Van Biesen W, Lameire N. What is the renal replacement method of first choice for intensive care patients? J Am Soc Nephrol. 2001;12:S40–S43.
- Ronco C, Cruz D, Bellomo R. Continuous renal replacement therapy in critical illness. Contrib Nephrol. 2007;156:309–319.
- Schetz M. Vascular access for IHD and CRRT. Contrib Nephrol. 2007;156:275–286.
- Wentling AG. Hemodialysis catheters: materials, design and manufacturing. Contrib Nephrol. 2004;142:112–127.
- Savader SJ, Haikal LC, Ehrman KO, Porter DJ, Oteham AC. Hemodialysis catheter-associated fibrin sheaths: treatment with a low-dose rt-PA infusion. J Vasc Interv Radiol. 2000;11:1131–1136.
- Oliver MJ, Mendelssohn DC, Quinn RR, . Catheter patency and function after catheter sheath disruption: a pilot study. Clin J Am Soc Nephrol. 2007;2:1201–1206.
- Grudzinski L, Quinan P, Kwok S, Pierratos A. Sodium citrate 4% locking solution for central venous dialysis catheters – an effective, more cost-efficient alternative to heparin. Nephrol Dial Transplant. 2007;22:471–476.
- Vanholder R. Vascular access: care and monitoring of function. Nephrol Dial Transplant. 2001;16:1542–1545.
- Schindler R, Heemann U, Haug U, . Bismuth coating of non-tunneled hemodialysis catheters reduces bacterial colonization: a randomized controlled trial. Nephrol Dial Transplant. 2010;25:2651–2656.
- Baumann M, Witzke O, Dietrich R, . Prolonged catheter survival in intermittent hemodialysis using a less thrombogenic micro-patterned polymer modification. ASAIO J. 2003;49:708–712.
- Meier P, Meier R, Turini P, Friolet R, Blanc E. Prolonged catheter survival in patients with acute kidney injury on continuous renal replacement therapy using a less thrombogenic micro-patterned polymer modification. Nephrol Dial Transplant. 2011;26:628–635.
- Parienti JJ, Thirion M, Mégarbane B, .; Members of the Cathedia Study Group. Femoral vs. jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA. 2008;299:2413–2422.
- Naka T, Egi M, Bellomo R, Baldwin I, Fealy N, Wan L. Resistance of vascular access catheters for continuous renal replacement therapy: an ex-vivo evaluation. Int J Artif Organs. 2008;31:905–909.
- Tan HK, Bridge N, Baldwin I, Bellomo R. An ex-vivo evaluation of vascular access catheters for continuous hemofiltration. Ren Fail. 2002;24:755–762.
- Unger JK, Haltern C, Portz B, Dohmen B, Gressner A, Rossaint R. Relation of hemofilter type to venous catheter resistance is crucial for filtration performance and hemocompatibility in CVVH – an in vitro study. Nephrol Dial Transplant. 2006;21:2191–2201.
- Unger JK, Pietzner K, Francis RC, . Dual lumen catheters for continuous veno-venous hemofiltration: limits for blood delivery via femoral vein access and a potential alternative in an experimental setting in anaesthetized pigs. Crit Care. 2007;11:R18.
- Parienti JJ, Mégarbane B, Fischer MO, .; Cathedia Study Group. Catheter dysfunction and dialysis performance according to vascular access among 736 critically ill adults requiring renal replacement therapy: a randomized controlled study. Crit Care Med. 2010;38:1118–1125.
- Brain M, Parkes S, Fowler R, Robertson I, Brown A. Calcium flux in continuous veno-venous hemofiltration with heparin and citrate anticoagulation. Crit Care Resusc. 2011;13:72–82.
- Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156.
- Agarwal B, Shaw S, Hari MS, Burroughs AK, Davenport A. Continuous renal replacement therapy in patients with liver disease: is circuit life different? J Hepatol. 2009;51: 504–509.
- Wright BJ. The RENAL replacement therapy study investigators. Intensity of continuous renal replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638.