Abstract
Matrix metalloproteinase-9 (MMP-9) is an enzyme that implicates in the pathogenesis of renal diseases. Henoch–Schönlein purpura nephritis (HSPN) is the most serious long-term complication of Henoch–Schönlein purpura, and it is one of the most common renal diseases in children. There was no any meta-analysis to explore the association of MMP-9 level with the risk of HSPN, and this meta-analysis was performed to evaluate the association between MMP-9 level and HSPN risk in children. A predefined literature search and selection of eligible relevant studies were performed to collect data from electronic databases. Eight articles were identified for the analysis of association between MMP-9 level and HSPN risk in children. In this meta-analysis, urinary MMP-9 level was associated with the risk of HSPN [odds ratio (OR) = 77.21, 95% confidence interval (CI): 54.56–99.86, p < 0.00001]. Furthermore, there was a notably difference for the association of the serum MMP-9 level with HSPN risk (OR = 135.91, 95% CI: 82.54–189.27, p < 0.00001). In conclusion, urinary/serum MMP-9 level is associated with the risk of HSPN. However, the data used for the meta-analysis in this article were from some low-level studies with small samples, and more studies should be performed in the future.
INTRODUCTION
Matrix metalloproteinase-9 (MMP-9), an important member of gelatinase, is a 92-kDa zinc-dependent endopeptidase that degrades components of the extracellular matrix (ECM).Citation1 Metabolize dysfunction of MMP-9 was reported to take part in the pathogenesis of renal diseases.Citation2–4 The homeostasis of ECM is destroyed, which is an important characteristic of renal diseases. MMP-9 is one of the most important MMPs for the ECM degradation. MMP-9 level might be an important factor in the pathogenesis of renal diseases.
Henoch–Schönlein purpura (HSP) is a systemic disorder characterized by leukocytoclastic vasculitis involving the capillaries and the deposition of IgA immune complexes.Citation5 Renal involvement, also called Henoch–Schönlein purpura nephritis (HSPN), is the principal cause of morbidity and mortality in children with HSP.Citation5 MMP-9 was reported to be associated with the onset of HSPN in the past decades. HSPN is characterized by cell proliferation and abnormal ECM remodeling by mesangial cells leading to fibrosis, sclerosis, and end-stage renal disease. MMP-9 is reported as the most important proteolytic enzyme involved in remodeling of ECM.Citation6,7 There might be an association between MMP-9 level and HSPN risk.
The evidence from meta-analysis might be powerful when compared with the individual investigation. There was no any meta-analysis performed to investigate the association between MMP-9 level and the risk of HSPN. This meta-analysis was performed to investigate the association of the MMP-9 expression with the susceptibility of HSPN in children.
MATERIALS AND METHODS
Search Strategy
The relevant studies were screened from the search engines of PubMed, Cochrane Library, and CBM-disc (China Biological Medicine Database) on 1 May 2012. In order to search the literature widely, the keyword of “Henoch–Schönlein purpura” was used instead of “Henoch–Schönlein purpura nephritis.” “(matrix metalloproteinase-9 OR MMP-9) AND (Henoch–Schönlein purpura OR HSP)” was used in those databases mentioned above. We also extended search spectrum to the “related articles” and the bibliographies of all retrieved studies. If multiple publications from the same study group using the same data occurred, we only recruited the later paper for analysis.
Inclusion and Exclusion Criteria
Inclusion criteria
(1) The study had to be about HSPN, (2) offering the data of level of MMP-9, and (3) study should be performed in children.
Exclusion criteria
(1) Review articles/case reports, (2) same data for multiple publications, and (3) investigating the association of MMP-9 with other diseases.
Data Extraction and Synthesis
The following information was extracted from each study independently by the investigators: author’s surname, year of publication, location of study, language of publication, level of MMP-9, and the number of subjects. The results were compared and disagreements were resolved by discussion.
Statistical Analyses
Available data were entered into Cochrane Review Manager (RevMan, Version 5, Oxford, UK) and analyzed. The pooled statistic was counted using the fixed effects model, but a random effects model was conducted when the p-value of heterogeneity test was less than 0.1.Citation8,9 Results were expressed with weighted mean differences (WMDs) for continuous data, and 95% confidence intervals (CIs) were also counted. A p value <0.05 was required for the overall odds ratio (OR) to be deemed statistically significant.Citation10,11 I2 was used to test the heterogeneity between the included studies.Citation12,13
RESULTS
Study Characteristics for the Relationship between MMP-9 Level with HSPN Risk
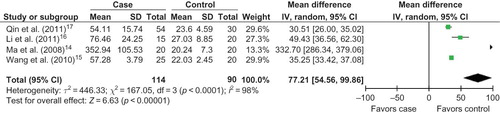
Four literaturesCitation14–17 were included into the meta-analysis for the relationship between urine MMP-9 level with the risk of HSPN in children (). All the four studies were performed in China, one published in English and three in Chinese. The average level of urine MMP-9 was 135.20 ± 37.33 in HSPN children, and 23.23 ± 5.80 in controls. The average level of urine MMP-9 in cases was much greater than that in controls (case/control = 5.82).
Table 1. Characteristics of the studies evaluating the effects of urine MMP-9 level on HSPN risk.
Table 2. Characteristics of the studies evaluating the effects of serum MMP-9 level on HSPN risk.
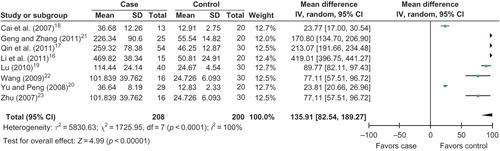
Eight literaturesCitation16–23 were included into the meta-analysis for the relationship between serum MMP-9 level with the risk of HSPN in children (). All the eight studies were performed in China, one published in English and seven in Chinese. The average level of serum MMP-9 was 168.36 ± 41.43 in HSPN children, and 31.56 ± 9.30 in controls. The average level of urine MMP-9 in cases was much greater than that in controls (case/control = 5.33).
Association of Urinary MMP-9 Level with HSPN Risk
Four studies were included into this meta-analysis. When the fixed effects model was used, there was a notable difference in the association of the urinary MMP-9 level with HSPN risk (OR = 35.22, 95%CI: 33.54–36.91, p < 0.00001). When the random effects model was conducted, we found that urinary MMP-9 level was associated with the risk of HSPN (OR = 77.21, 95% CI: 54.56–99.86, p < 0.00001; ). The result of the meta-analysis for the association of urinary MMP-9 level with HSPN risk was robust to some extent.
Association of Serum MMP-9 Level with HSPN Risk
Eight reports were included into the meta-analysis. When the fixed effects model was used, we found that serum MMP-9 level was associated with the risk of HSPN (OR = 42.01, 95% CI: 39.42–44.59, p < 0.00001). When the random effects model was conducted, there was a notably difference for the association of the serum MMP-9 level with HSPN risk (OR = 135.91, 95% CI: 82.54–189.27, p < 0.00001; ). The result of the meta-analysis for the association of serum MMP-9 level with HSPN risk was robust to some extent.
DISCUSSION
In this meta-analysis, we found that urine MMP-9 and serum MMP-9 levels were associated with the risk of HSPN, and urine/serum MMP-9 might be an important marker for the onset of HSPN. There was significant heterogeneity among the included studies. The final results originated from the model of random effects model. We also performed the meta-analysis using fixed effects model, and also found that urine MMP-9 and serum MMP-9 levels were associated with the risk of HSPN. The conclusion form our meta-analysis might be robust to some extent.
Until now, most of the studies reporting the association of MMP-9 with the risk of HSPN have been performed in China and were published in Chinese. There was only one study reported in English publication. More studies should be performed in the English country and reported in English in the future.
In the past decades, Danilewicz et al.Citation7 determined the glomerular immunoexpression of MMP-9 in IgA nephropathy (IgAN) patients and HSPN patients and found that glomerular staining for MMP-9 did not differ between these two groups. They also reported that the glomerular staining of MMP-9 was not significantly positively correlated with mesangial cells as well as glomerular alpha-smooth muscle actin staining in both HSPN and IgAN. However, there were some reports which found that MMP-9 was associated with the risk of HSP. Shin et al.Citation24 investigated the gene expression profile of MMP-9 in children with HSP and examined the role of MMP-9 in the pathogenesis of HSP and found that MMP-9 level was significantly higher in patients in the acute stage of HSP than in normal controls. Mahajan et al.Citation25 conducted a study to investigate the potential role of MMP-9 in HSP and reported that the activity and expression of MMP-9 in serum were marked increased when compared with those in healthy controls. Zou et al.Citation26 performed a study to investigate the potential role of MMP-9 in HSP and reported that plasma MMP-9 level in the acute phase of HSP was significantly higher than that in healthy children, and immunocytochemistry showed that MMP-9 was positive in the circulating white blood cells of HSP children. MMP-9 level might be associated with the risk of HSP in the present publication in English. More studies should be performed to confirm this role of MMP-9 further.
MMP-9 might be a useful indicator to predict the risk of HSPN. Qin et al.Citation17 found that the optimal cut-off point (sensitivity; specificity) of serum MMP-9 for diagnosing HSPN was 179.79 mg/L (0.96; 0.88). The serum MMP-9 might predict the onset of HSPN in children. However, more studies should be performed to confirm it.
Abnormal ECM remodeling is an important characteristic for the glomerulus lesion of HSPN patient. MMP-9 is regarded as the most important proteolytic enzymes involved in the degradation of ECM.Citation7 The increased serum MMP-9 would degrade the ECM and break the integrality of basement membrane in glomerulus. The serum MMP-9 might pass through the basement membrane and the urinary MMP-9 level might be increased.
Some limitations should be declared in this meta-analysis. First, heterogeneities might be present, and it might affect the conclusion of our meta-analysis, although a random effects model has been conducted. Second, an important threat to any literature-based review and meta-analysis is that of reporting bias (only recruit published literatures in English and Chinese). Last but not least, the sample sizes in some studies are relatively small. Undoubtedly, the limitations mentioned above might affect our final conclusions.
In conclusion, the results in our study supported that urine/serum MMP-9 was associated with the susceptibility of HSPN in children. However, the data used for meta-analysis in this article were from some low-level studies with small samples. More association investigations on larger populations are required to further clarify the role of urinary/serum MMP-9 in HSPN susceptibility in children.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Mittelstadt ML, Patel RC. AP-1 mediated transcriptional repression of matrix metalloproteinase-9 by recruitment of histone deacetylase 1 in response to interferon beta. PLoS One. 2012;7:42152.
- Wang X, Zhou Y, Tan R, . Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2010;299:973–982.
- Yao XM, Ye SD, Zai Z, . Simvastatin protects diabetic rats against kidney injury through the suppression of renal matrix metalloproteinase-9 expression. J Endocrinol Invest. 2010;33:292–296.
- Yao XM, Ye SD, Chen Y, . Rosiglitazone protects diabetic rats against kidney injury through the suppression of renal matrix metalloproteinase-9 expression. Diabetes Obes Metab. 2009;11:519–522.
- Kawasaki Y. The pathogenesis and treatment of pediatric Henoch–Schonlein purpura nephritis. Clin Exp Nephrol. 2011;15:648–657.
- Zhou TB, Qin YH. The potential mechanism for the different expressions of gelatinases induced by all-trans retinoic acid in different cells. J Recept Signal Transduct Res. 2012;32:129–133.
- Danilewicz M, Wagrowska-Danilewicz M. Differential glomerular immunoexpression of matrix metalloproteinases MMP-2 and MMP-9 in idiopathic IgA nephropathy and Schoenlein-Henoch nephritis. Folia Histochem Cytobiol. 2010;48:63–67.
- Zhou TB, Qin YH, Su LN, . Insertion/deletion (I/D) polymorphism of angiotensin-converting enzyme gene in steroid-resistant nephrotic syndrome for children: a genetic association study and meta-analysis. Ren Fail. 2011;33:741–748.
- Zhou TB, Ou C, Qin YH, . Association of angiotensin converting enzyme insertion/deletion gene polymorphism with idiopathic nephrotic syndrome susceptibility in children: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011;12:601–610.
- Zhou TB, Liu YG, Lin N, . Relationship between angiotensin-converting enzyme insertion/deletion gene polymorphism and systemic Lupus Erythematosus/Lupus Nephritis: a systematic review and metaanalysis. J Rheumatol. 2012;39:686–693.
- Zhou TB, Qin YH, Ou C, . A meta-analysis of the association between angiotensin-converting enzyme insertion/deletion gene polymorphism and steroid-sensitive nephrotic syndrome in children. J Renin Angiotensin Aldosterone Syst. 2012;13:175–183.
- Zhou TB, Qin YH, Su LN, Lei FY, Huang WF, Zhao YJ. ACEI/D gene polymorphism can’t predict the steroid responsiveness in Asian children with idiopathic nephrotic syndrome: a meta-analysis. PLoS One. 2011;6:19599.
- Zhou TB, Qin YH, Xu HL. Association of apoE gene expression and its gene polymorphism with nephrotic syndrome susceptibility: a meta-analysis of experimental and human studies. Mol Biol Rep. 2012;39:9347–9354.
- Ma H, Lin H, Ma G. Urinary levels of collagen IV and MMP-9 and their clinical significances in children with Henoch–Schonlein purpur. Med Innov Res. 2008;5:8–9.
- Wang S, Qi X, Pan T, Xu L. The relationship between urinary microprotein and metalloproteinase-9 expression in children with Nephritis of Schonle–Henoch Purpura. J Hubei Univ Med. 2010;29:515–517.
- Li C, Chen Q, Zhang Y, Wang M, Ke P. Changes and clinical significance of matrix metalloproteinase-9 in children with Henoch–Schonlein purpura nephrin. Guangdong Med J. 2011;32:2945–2946.
- Qin YH, Zhou TB, Lei FY, . Cut-off values for serum matrix metalloproteinase-9: is there a threshold to predict renal involvement for Henoch–Schonlein purpura in children? Nephrology (Carlton). 2011;16:93–99.
- Cai W, Li A, Chen S, Li C, Cui H. Clinical significance of the change of matrix metalloproteinase-9 children with Henoch–Schönlein purpura nephritis. Shandong Med J. 2007;47:42–43.
- Lu Y. The clinical significance of the detection of matrix metalloproteinase 9 in children with Henoch–Schönlein purpura nephritis. Mod Pract Med. 2010;22:1178–1180.
- Yu Z, Peng X. The changes and clinical significance of IL-6, IL-8, MMP-9 in serum of children with Henoch–Schönlein purpura nephritis. Hebei Med. 2008;14:573–576.
- Geng XZ, Zhang H. Relationship between fmatrix metalloproteinase level and kidney damage in children with Henoch–Schonlein purpura nephritis. J Henan Univ Sci Tech (Med Sci). 2011;29:100–102.
- Wang YB, AS. Matrix metalloproteinase 2, 9 and its tissue inhibitor 1 expression and their significance in the Henoch–Schönlein purpura nephritis. Harbin Med J. 2009;29:13–14.
- Zhu QY, Jiang YH, Liu DJ, Sun Q. Significance of changes of serum levels and ratios of matrix nephritis metalloproteinase - 2, - 9 and tissue inhibitor of metalloproteinase-1 in children with Henoch–Schönlein purpura nephritis. J Appl Clin Pediatr. 2007;22:343–344.
- Shin JI, Song KS, Kim H, . The gene expression profile of matrix metalloproteinases and their inhibitors in children with Henoch–Schonlein purpura. Br J Dermatol. 2011;164:1348–1355.
- Mahajan N, Bisht D, Dhawan V, Singh S, Minz RW. Transcriptional expression and gelatinolytic activity of matrix metalloproteinases in Henoch-Schonlein purpura. Acta Paediatr. 2010;99:1248–1252.
- Zou CC, Zhao ZY, Tang LF, Liang L. Plasma levels of matrix metalloproteinase-9 in Henoch-Schonlein purpura. Scand J Rheumatol. 2006;35:52–55.