Abstract
Anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis is a rare complication of antithyroid drug use that was first described with propylthiouracil. We describe an ANCA-associated rapidly progressive glomerulonephritis in a patient treated with carbimazole during 6 months for Graves disease that resulted in end-stage renal disease. A 66-year-old man treated with carbimazole for Graves disease was admitted for macroscopic hematuria and edema of the lower extremities. Laboratory work-up showed elevated serum creatinine (435 μmol/L), mixed hematuria, nephrotic range proteinuria, and a low positive c-ANCA titer with proteinase-3 specificity. Renal biopsy showed necrotizing, crescentic, pauci-immune glomerulonephritis. Carbimazole was discontinued and hemodialysis was initiated as well as high-dose glucocorticoids and pulses of intravenous cyclophosphamide. Despite immunosuppressive treatment, the patient remained dialysis-dependent at 6 months after diagnosis. Graves disease remained in remission after carbimazole withdrawal. ANCA-associated vasculitis manifesting as glomerulonephritis is a potential adverse effect of all antithyroid drugs. Although prognosis is usually good, end-stage renal disease may ensue in rare cases. Physicians should have a high index of suspicion in patients receiving antithyroid drugs who present with symptoms or signs suggestive of progressive renal disease.
INTRODUCTION
Antithyroid drugs for Graves disease include carbimazole, methimazole, propylthiouracil (PTU), and benzyl thiouracil. Common side effects are headache, arthralgias, and cutaneous manifestations. Between the rare but severe adverse effects, the best described is agranulocytosis.
A rare complication of antithyroid drug use is anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis. It was first described with PTU, then with other antithyroid agents. An ANCA positive titer is a common finding in PTU-treated patients, but its significance remains uncertain, as most of them have no symptoms.Citation1 However, ANCA levels should be carefully evaluated in patients with clinical presentation suggestive of vasculitis.
Here, we describe an ANCA-associated rapidly progressive glomerulonephritis in a patient treated with carbimazole for Graves disease that resulted in end-stage renal disease.
CASE REPORT
A 66-year-old man was admitted because of macroscopic hematuria and edema of the lower extremities. There was no history of hemophthisis, nausea, vomiting, or diarrhea before admission. He had history of Child B cirrhosis in the setting of alcohol abuse (diagnosed the previous year), sufficiently controlled hypertension, major depression, Graves disease, and insulin-requiring type 2 diabetes mellitus diagnosed in the ambulatory setting several years ago. The degree of diabetes control was satisfactory (HbA1c 7.6%). The patient did not have diabetic retinopathy. Baseline creatinine levels were in the normal range (93 μmol/L). He had microalbuminuria (albumin to creatinine ratio of 20 mg/mmol). He had no history of nephrolithiasis.
Graves disease was suspected 6 months ago because of insomnia and tachycardia and was confirmed by thyroid-stimulating hormone (TSH) levels <0.004 mU/L (normal range 0.4–4 mU/L), free thyroxine (FT4) 40.8 pmol/L (normal range 10.3–23.8 pmol/L), total triiodothyronine (T3) 4.1 nmol/L (normal range 0.8–2.7 nmol/L), and positive TSH receptor antibody (TRAB) titer at 10.5 U/L (normal range <1U/L). The patient was treated with carbimazole 30 mg per day, then 10 mg per day. His usual treatment also included glargine and aspart insulin, escitalopram, mirtazapine, oxazepam, metoprolol, torsemide, lisinopril, amlodipine, and complex B vitamins.
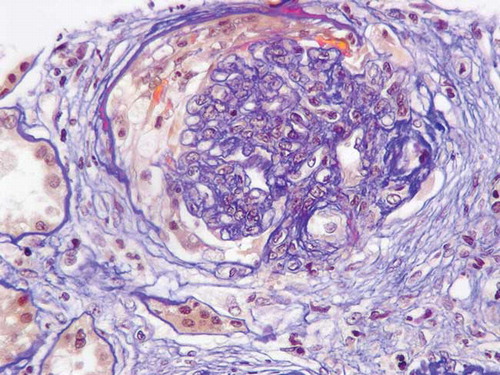
On admission, the patient was alert, body temperature was 36°C, and blood pressure was 140/70 mm Hg, pulse rate 70 per minute and respiratory rate 12 per minute. A 3/6 systolic murmur was audible at the base and pitting edema of the lower extremities was present. Urine output was normal. Laboratory work-up showed: hemoglobin 82 g/L, platelets 125 G/L, erythrocyte sedimentation rate 110 mm/h, C-reactive protein 94 mg/L, serum creatinine 435 μmol/L, Na+ 128 mmol/L,K+ 4.6 mmol/L, aspartate transaminase 19U/L, alanine transaminase 27 U/L, Ca++ 2.4 mmol/L, phosphate 2.1 mmol/L, urea 17 mmol/L, glucose 5.3 mmol/L, total protein 59 g/L, albumin 21 g/L. Urinalysis showed mixed hematuria (glomerular and non-glomerular), nephrotic range proteinuria (528 mg/mmol creatinine) and hyaline casts. Urine cultures were sterile. Antinuclear antibody (ANA) assay was negative, C3 and C4 levels normal, IgG and IgA levels elevated, and IgM levels in the normal range without monoclonal peak. A low positive c-ANCA titer was present with proteinase-3 (PR3) specificity.Thyroid function tests were suggestive of hyperthyroidism responding to treatment: TSH 0.01 mU/L, FT4 13.9 pmol/L, and total T3 <0.62 nmol/L. Ultrasonography of the urinary tract ruled out obstruction. Renal biopsy on day 4 from admission () showed necrotizing crescentic pauci-immune glomerulonephritis (five cellular and two fibrotic crescents out of 20 glomeruli, one glomerulus with focal segmental glomerulosclerosis, ischemic lesions in the tubuli). Work-up for Wegener disease was negative (no upper airway involvement on the evaluation by an ear–nose–throat specialist, no pulmonary lesions on CT scan, normal neurological status, normal ECG). There were no predisposing factors for hepatorenal syndrome before admission. Besides carbimazole, no other medication was recently introduced. The diagnosis of ANCA-associated rapidly progressive glomerulonephritis induced by carbimazole treatment was retained.Carbimazole was immediately discontinued.
Creatinine rapidly increased at 693 μmol/L in a week and hemodialysis was initiated (10th day of hospitalization) as well as high-dose glucocorticoids (7th day) and pulsed intravenous cyclophosphamide (10th day). Cyclophosphamide (500 mg/m2) was administered in 250 mL of 5% glucose in 1 h with 400 mg of MESNA, this dose adjusted for the age of the patient. Cyclophosphamide pulses were programmed at a frequency of twice per month the first months then monthly, but the patient received only the first dose since he subsequently developed infectious complications (erysipelas of the leg, intravascular catheter-associated bacteremia).
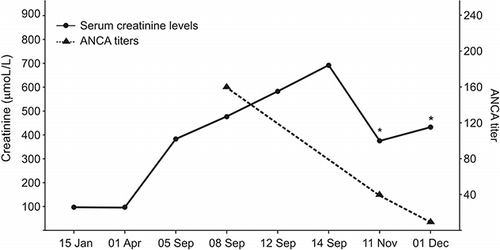
After discontinuing cyclophosphamide, the patient received prednisone at 1 mg/kg for 3 weeks. Prednisone was then gradually tapered up to 15 mg (by 10 mg increments every 3 weeks). Despite this, the patient remained dialysis-dependent 6 months after diagnosis. However, ANCA titer became negative 4 months after diagnosis. Free T4 levels were stable in the normal range after stopping carbimazole. shows the evolution of creatinine levels and ANCA titers. No other immunosuppressive therapy was undertaken since the patient presented recurrent life-threatening infectious events.
Figure 1. Serum creatinine levels and ANCA titer on different occasions. Note that creatinine levels were in the normal range before starting carbimazole (01 April). Immunologic remission was achieved after carbimazole withdrawal (ANCA titer non-detectable).Note: *Patient on intermittent renal replacement therapy.
DISCUSSION
This is the third reported case of carbimazole-induced, ANCA-associated, crescentic glomerulonephritis, but the first one associated with PR3 ANCA and the first one resulting in dialysis-dependent chronic kidney disease.Citation2,Citation3 Carbimazole has also been described once to cause dialysis-dependent chronic kidney disease secondary to interstitial nephritis.Citation4 However, in that case, ANCA were negative and there was no evidence of glomerulonephritis or vasculitis in the renal biopsy.
ANCA-associated vasculitis with renal involvement is a rare adverse effect in patients treated with antithyroid drugs. Its natural history may be less severe compared with idiopathic vasculitis.Citation5 Most cases are associated with PTU. Genetic predisposition has a major role for the development of this adverse effect.Citation6
illustrates the reported cases of antithyroid drug-induced, ANCA-associated vasculitis with renal involvement. Several other cases, all concerning PTU, have also been reported in the Japanese literature and were reviewed by Gunton et al.Citation7 Myeloperoxidase (MPO) and PR3 ANCA-specific antigens are more commonly encountered. End-stage renal disease necessitating hemodialysis has twice been reported with patients remaining dialysis-dependent.Citation8,Citation9 However, in most cases, prognosis is good as renal failure resolves with specific treatment. Death has once been reported and was due to acute diffuse alveolar hemorrhage.Citation10 In our patient, pre-existing comorbidities (mostly liver cirrhosis and diabetes) and premature interruption of cyclophosphamide treatment due to infectious complications may explain the poor outcome.
Table 1. Antithyroid, drug-induced, ANCA-associated glomerulonephritis: literature review.
Cessation of the incriminated antithyroid drug is essential and usually sufficient to attain remission.Citation11 However, in case of severe organ involvement, intensive corticosteroids and immunosuppressive therapy should be administered.Citation7 Plasmapheresis is the treatment of choice in the presence of pulmonary hemorrhage. We did not consider plasmapheresis in our patient because of the liver cirrhosis history (considered as an immunosuppressed patient) and the concomitant infectious complications.
Hyperthyroidism control in the setting of antithyroid, drug-induced, ANCA-associated vasculitis may be problematic. Different approaches proposed in the literature are thyroidectomyCitation2 or switch to another antithyroid drug with subsequent radioactive iodine therapy.Citation12 Iodine solution administration may also be an option in patients awaiting thyroidectomy.Citation13 In our patient, thyroid function was closely monitored after carbimazole discontinuation. It is interesting that Graves disease remained in remission in a patient who received carbimazole for only 6 months and was slightly hyperthyroid under 10 mg of carbimazole per day. Concomitant immunosuppressive treatment possibly contributed to remission.
In conclusion, ANCA-associated vasculitis is a potential complication of all antithyroid drugs that may rarely cause end-stage renal disease. Monitoring patients with urinary stix and serum creatinine during the first months of treatment and then on a yearly basis could be suggested. Physicians should have a high index of suspicion in patients receiving antithyroid drugs who present with symptoms or signs suggestive of progressive renal disease in order to immediately withdraw the responsible agent and proceed to adequate diagnostic work-up and treatment.
ACKNOWLEDGMENT
We thank S. Moll, MD, and D. Adamopoulos, MD, PhD, for their expert assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Sera N, Ashizawa K, Ando T, . Treatment with propylthiouracil is associated with appearance of antineutrophil cytoplasmic antibodies in some patients with Graves’ disease. Thyroid. 2000;10:595–599.
- Calañas-Continente A, Espinosa M, Manzano-García G, Santamaria R, Lopez-Rubio F, Aljama P. Necrotizing glomerulonephritis and pulmonary hemorrhage associated with carbimazole therapy. Thyroid. 2005;15:286–288.
- D’Cruz D, Chesser AM, Lightowler C, . Antineutrophil cytoplasmic antibody-positive crescentic glomerulonephritis associated with anti-thyroid drug treatment. Br J Rheumatol. 1995;34:1090–1091.
- Day C, Bridger J, Rylance P, Jackson M, Nicholas J, Odum J. Leukocytoclastic vasculitis and interstitial nephritis with carbimazole treatment. Nephrol Dial Transplant. 2003;18:429–431.
- Bonaci-Nikolic B, Nikolic MM, Andrejevic S, Zoric S, Bukilica M. Antineutrophil cytoplasmic antibody (ANCA)-associated autoimmune diseases induced by antithyroid drugs: comparison with idiopathic ANCA vasculitides. Arthritis Res Ther. 2005;7:R1072–R1081.
- Herlin T, Birkebaek NH, Wolthers OD, Heegaard NH, Wiik A. Antineutrophil cytoplasmic antibodies (ANCA) prophiles in propylthiouracil-induced lupus-like manifestations in monozygotic triplets with hyperthyroidism. Scand J Rheumatol. 2002;31:46–49.
- Gunton JE, Stiel J, Caterson RJ, McElduff A. Clinical case seminar: anti-thyroid drugs and antineutrophil cytoplasmic antibody positive vasculitis. A case report and review of the literature. J Clin Endocrinol Metab. 1999;84:13–16.
- Gao Y, Chen M, Ye H, Yu F, Guo XH, Zhao MH. Long-term outcomes of patients with propylthiouracil-induced anti-neutrophil cytoplasmic auto-antibody-associated vasculitis. Rheumatology. 2008;47:1515–1520.
- Vogt BA, Kim Y, Jennette JC, Falk RJ, Burke BA, Sinaiko A. Antineutrophil cytoplasmic autoantibody-positive crescentic glomerulonephritis as a complication of treatment with propylthiouracil in children. J Pediatr. 1994;124:986–988.
- Batchelor N, Holley AA. Fatal case of propylthiouracil-induced ANCA-positive vasculitis. MDMedGenMed. 2006;8:10.
- Wiik A. Clinical and laboratory characteristics of drug-induced vasculitic syndromes. Arthritis Res Ther. 2005;7:191–192.
- Tieulie N, Huong DL, Andreu M, . ANCA associated glomerulonephritis related to benzylthiouracil. Rev Med Int. 2002;23:853–856.
- Trimeche Ajmi S, Braham R, Toumi S, . Benzylthiouracil-induced glomerulonephritis. Case Report Med. 2009;2009:687285.
- Jarraya F, Abid M, Jlidi R, . Myeloperoxidase-antineutrophil cytoplasmic antibody-positive crescentic glomerulonephritis associated with benzylthiouracil therapy: report of the first case. Nephrol Dial Transplant. 2003;18:2421–2423.
- Kaaroud H, Khiari K, Ben Moussa F, Barbouch S, Boussema E, Ben Maïz H. Vasculitis with renal and pulmonary involvement in a patient receiving benzyl thiouracil for Graves disease. Rev Med Int. 2002;23:857–861.
- Erten Y, Bodur H, Sahiner S, Akkaya V, Canbakan B, Bali M. Antineutrophil cytoplasmic antibody associated vasculitis and rapidly progressive glomerulonephritis as a complication of propylthiouracil therapy. Clin Endocrinol. 2002;57:699–700.
- Winters MJ, Hurley RM, Lirenman DS. ANCA-positive glomerulonephritis and IgA nephropathy in a patient on propylthiouracil. Pediatr Nephrol. 2002;17:257–260.
- Hori Y, Arizono K, Hara S, Kawai R, Hara M, Yamada A. Antineutrophil cytoplasmic autoantibody-positive crescentic glomerulonephritis associated with thiamazole therapy. Nephron. 1996;74:734–735.
- Yuksel C, Ayli MD, Yuksel A, . Propylthiouracil-induced vasculitis associated with ANCA: a case report. Renal Fail. 2007; 29:235–237.
- Braham A, Houman MH, Rais L, Ben Gborbel I, Lamloum M, Miled M. Benzylthiouracil induced ANCA-positive vasculitis. Presse Med. 2004;33:1331–1333.
- Tanemoto M, Miyakawa H, Hanai J, Yago M, Kitaoka M, Uchida S. Myeloperoxidase-antineutrophil cytoplasmic antibody-positive crescentic glomerulonephritis complicating the course of Graves’ disease: report of three adult cases. Am J Kidney Dis. 1995;26:774–780.