Abstract
There is an increasing evidence that oxidative stress plays an important role in the pathogenesis of rhabdomyolysis-induced acute renal failure (ARF). In this study, protective effects of L-citrulline on glycerol-induced ARF in rats were investigated. Six groups of rats were employed in this study: group 1 served as a control; group 2 was only given glycerol (50%, 10 mL/kg, i.m.); group 3 was given glycerol plus dexamethasone (0.1 mg/kg, i.g.) as positive reference drug, starting at the same time as the glycerol injections; the last three groups were given glycerol plus L-citrulline (300, 600, and 900 mg/kg, i.g.) respectively, starting at the same time as the glycerol injections. The injections of glycerol were only once, and after glycerol injections the i.g. administrations of dexamethasone and L-citrulline were repeated every 24 h for 7 days. After 7 days of glycerol injections, the blood samples and kidney tissues were harvested for future biochemical and pathology analyses. The levels of creatinine (Cr) and urea nitrogen (BUN) in plasma, the content of malondialdehyde (MDA), glutathione (GSH), nitric oxide (NO), the activity of total nitric oxide synthase (TNOS), inducible nitric oxide synthase (iNOS), endothelial NO synthase (eNOS), and superoxide dismutase (SOD) were evaluated in kidney tissues. Consequently, administrations of L-citrulline improved an impaired intrarenal oxygenation and kidney function compared with the glycerol group, and prevented the renal oxidative stress damage as well as severe functional and morphological renal deterioration. Therefore, L-citrulline might have potential application in the amelioration of glycerol-induced ARF.
INTRODUCTION
The pathogenesis of glycerol-induced myoglobinuric acute renal failure (ARF) involves vascular congestion, direct myoglobin-induced cytotoxicity, and reactive oxygen metabolites.Citation1 The striated muscle dissolved because of the intramuscular injection of glycerol, the myoglobin, and other potentially toxic intracellular components released into the systemic circulation. Rhabdomyolysis can be traumatic (muscle crush and/or pressure injury, extreme physical exertion) or non-traumatic in nature, and a great many of patients who are suffering from severe rhabdomyolysis develop some degree of ARF, some even have severe acute tubular necrosis.Citation2
The mechanisms of glycerol-induced myoglobinuric ARF are renal vasoconstriction and congestion, ischemia, and myoglobin-induced cytotoxicity.Citation3,4 Moreover, some studies show that the release of myoglobin lead to the increase of iron content in the plasma and the latter promotes free radical formation lipid peroxidation and renal dysfunction.Citation5,6 And there is growing evidence indicating that the reactive oxygen species (ROS) plays an important role in the pathogenesis of myoglobinuric ARF, oxidative stress would participate in the tubular necrosis favoring lipid peroxidation of cellular membranes.Citation1,7–9
In the kidney, NO is an important molecule in the regulation of renal microvascular tone, and much evidence suggests that intrarenal oxygen supply is NO, inappropriate high release of NO related to the tubular injury, inhibition of the renal endothelial NO synthase (eNOS), therefore the main production of NO is iNOS, an enzyme which can be blocked by dexamethasone.Citation10,11 Dexamethasone is glucocorticosteroid which have been advocated for decades, and it can inhibit the production of iNOS by attenuation in gene expression.Citation12 Some studies also showed that the dexamethasone has beneficial effect on renal dysfunction.Citation13,14 In this study, we use dexamethasone as positive reference drug to investigate the underlying mechanism of the protective effects of L-citrulline on glycerol-induced ARF.
L-Citrulline is as a free amino acid in plasma and urine, and an intermediate product of urea cycle. Much evidence suggests that L-citrulline can decrease suppress oxidative damage induced by exhaustive exercise.Citation15 Moreover, L-citrulline can become the effective precursor of L-arginine which is a substrate for endothelial NO synthase (eNOS) via the L-citrulline/L-arginine pathway, and plays an important role in the metabolism and regulation of NO.Citation16 Others demonstrated that oral supplementation of L-citrulline can inhibition the increased inducible nitric oxide synthase (iNOS) activity, upregulate endothelial NO synthase (eNOS) activity, and improve endothelial function in animal.Citation17–19
The purpose of this present study was to evaluate the protective effects of L-citrulline supplementation on the glycerol-induced ARF in rats, and its underlying mechanism.
MATERIALS AND METHODS
Animals
Thirty-six Sprague–Dawley rats weighing 180–230 g were used in the study, provided by the Experimental Animal Centre of Xuzhou Medical College. The animals were housed under a standard light/dark cycle with free access to food and water, the temperature (23 ± 1°C), and relative humidity (65%) was kept constant. All experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drugs
Glycerol was purchased from Jiangxi Yipusheng Company, China. L-Citrulline was purchased from HAIDE BIOCHME CO. LTD, dexamethasone was purchased from Jiangsu Lianshui Pharmaceutical Company, China, and the grade of other chemicals and reagents were analytical.
Experimental Design
Rats were divided into six groups randomly, and before the injections of glycerol the rats had free access to food but lacking drinking water for 24 h. Group 1 is control group (n = 6) and received intramuscularly equivalent volume of saline solution for glycerol in each hind leg.Citation7 Group 2 (n = 6) were intramuscularly injected with 50% glycerol (10 mL/kg) in each hind leg. Group 3 (n = 6) were intramuscularly injected with 50% glycerol (10 mL/kg) in each hind leg and orally received dexamethasone (0.1 mg/kg) starting at the same time as the glycerol injections. Groups 4, 5, and 6 were intramuscularly injected with 50% glycerol (10 mL/kg) in each hind leg and orally received L-citrulline (300, 600, and 900 mg/kg, respectively) starting at the same time as the glycerol injections. After glycerol injections, the i.g. administrations of dexamethasone and L-citrulline were repeated every 24 h for 7 days.
After 7 days, the animals were sacrificed and the blood samples were obtained with no addition of anticoagulants for centrifugation at 3000 × g for 10 min for subsequent determination of clear serum, and the bilateral nephrectomy was performed rapidly. One of the two kidneys was immersed in 4% formaldehyde for histological evaluation and the other was stored at −80°C for biochemical determinations.
Assessment of the Renal Function
Biochemical analyses of blood urea nitrogen (BUN) and creatinine (Cr) levels were made using the standard diagnostic kits (Nanjing Jiancheng Bioengineering Institute, China).
Malondialdehyde Assays
The level of MDA in the kidney tissue designated as an index of lipid peroxidation was measured according to the modified method of Ohkawa’s et al.Citation20 Kidney tissues was weighed and homogenized in 10 mL KCl (10%). The homogenate was supplemented with 8.1% sodium lauryl sulfate, 20% acetic acid, and 0.8% TBA, and was thereafter boiled at 100°C for 1 h. After cooling, the reactants were supplemented with 2.5 mL n-butanol for vigorous agitation for 1 min and centrifugation for 10 min. Absorbance was measured at 532 nm and the results were expressed as nmol TBA/mg of protein.
SOD Activity
The SOD activity was assayed by the method of Liu’s.Citation19 SOD evaluation was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase, which reacted with nitro-blue tetrazolium (NTB) to form formazan dye. SOD activity was then read at 550 nm by the degree of inhibition of this reaction. One unit of enzyme was defined as the amount of enzyme required at the inhibition rate of 50%. The activity of SOD was expressed as unit/mg protein.
GSH Level
The concentration of GSH was measured by the procedures of Ellman’sCitation21 Briefly, 0.5 mL homogenate was mixed with 1.5 mL 0.15 M KCl and 3 mL deproteinization solution. Each sample was centrifuged at 3000 rpm for 10 min and the supernatant was removed, followed by the addition of 2 mL phosphate solution and 0.5 mL DTNB into the 0.5 mL supernatant, with the absorbance read at 412 nm and compared with glutathione standards.
Nephritic NO Level
The content of NO in kidney was measured with the method of MAHMOUD M’s.Citation22 The kidney tissue of each rat was scrubbed off, weighed, and its nitric oxide content was determined as nitrite by diazotization with sulfanilic acid at acidic pH and subsequent coupling with N-1-naphthyl-ethylene diamine to give a colored product that was measured colorimetrically at 548 nm.
Nephritic NOS Activity
The measurement of NOS activity was according to the method of CAROL MORENO’s. The frozen tissues were homogenized in a solution containing 320 mM sucrose, 50 mM Trizma base, 1 mM EDTA, 1 mM d,l-dithiothreitol, 10 μg/mL leupeptin, 100 μg/mL phenylmethyl sulfonyl fluoride, 10 μg/mL soybean trypsin inhibitor, and 2 μg/mL aprotinin brought to pH 7 with HCl. Following centrifugation of the homogenate at 100,000 × g for 1 h, the supernatants were added to the reaction mixture containing 50 mmol/L Tris (pH 7.4), L-[U-14C]arginine (specific activity 11.8 GBq/mmoL), 10 μg/mL calmodulin, 1 mmol/L CaCl2, and 50 mmol/L L-valine. The samples were incubated for 20 min at 37°C prior to the termination of the reaction by the addition of 0.1 vol. of 20% (v/v) HClO4. L-[U-14C]Citrulline was isolated from L-[U-14C]arginine by passage through Dowex 50W (Na+form, Sigma–Aldrich, St Louis, MO, USA) and quantified by liquid-scintillation counting. The level of iNOS activity was measured by the addition of 1 mmol/L EGTA and TNOS activity by the addition of 2 mmol/L L-NMMA. The eNOS activity was determined by subtraction of iNOS activity from TNOS activity. Protein concentration of the supernatant was determined by spectrophotometry using a commercial assay kit (BioRad Laboratories, Richmond, CA, USA).
Kidney Morphological Studies
The right kidney was isolated immediately after sacrificing the animal and fixed in 10% formalin solution. And the kidney tissues were embedded in paraffin, 4 μm sections were stained with hematoxylin and eosin to evaluate the kidney morphology.
STATISTICAL ANALYSIS
All data were expressed as means ± SEM (standard error of the mean). The data were evaluated with SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The data were analyzed by one-way ANOVA, followed by Dunnett’s t-test. Statistical significance was set at p < 0.05.
RESULTS
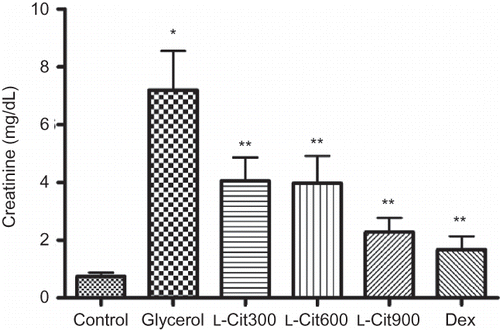
As shown in and , glycerol administration resulted in a significant increase in urea (p < 0.01) and creatinine (p < 0.01) levels compared with the control, and the rats treated with L-citrulline and dexamethasone in addition to glycerol had a significantly lower blood urea and creatinine than those receiving glycerol alone.
Figure 1. Effects dexamethasone (Dex) of L-Citrulline (L-Cit) on levels of BUN in rats plasma. Data are the means ± SEM for six animals. Note: *p < 0.01 as compared to the control, **p < 0.05 as compared to the glycerol group, ***p < 0.01 as compared to the glycerol group.
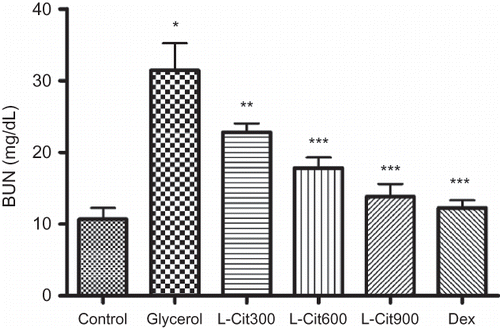
Figure 2. Effects dexamethasone (Dex) of L-Citrulline (L-Cit) on levels of Cr in rats plasma. Data are the means ± SEM for six animals.Note: *p < 0.01 as compared to the control, **p < 0.01 as compared to the glycerol group.
MDA is an index of lipid peroxidation, the level of MDA in kidney tissue significantly increased to 0.760 ± 0.008 nmol TBA/mg protein in rats treated with glycerol as compared to the control. But this increase was not significant in animals with L-citrulline gavage. The activity of the antioxidant enzymes SOD and the content of GSH were significantly decreased compared to the receiving glycerol alone (p < 0.01), however, the administrations of L-citrulline protected the kidney against the loss of the antioxidant enzyme activity, resulting in a significant increase in enzymatic SOD and GSH activities approximate to the control levels (see ).
Table 1. Effect of L-citrulline on SOD and GSH and MDA levels in the kidney tissue of rats administered with glycerol.
Table 2. Effect of L-citrulline on NOS activity in the kidney tissue of rats administered with glycerol.
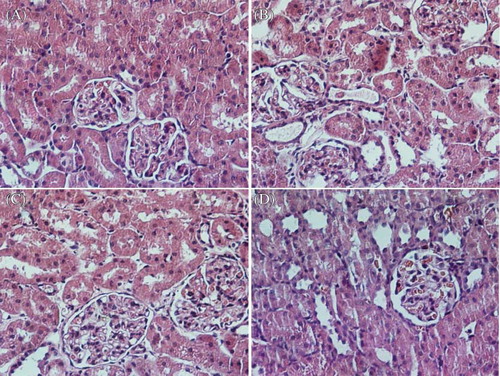
Microphotographies of the kidney in each group were shown in Figure 3A, B, C and D. The morphological study of the kidney of normal animals showed no damage (A). The kidneys obtained from glycerol-injected rats showed epithelial necrosis, tubular dilation (B). The renal tubules of rats in the glycerol + L-citrulline (900 mg/kg) and glycerol + dexamethasone group showed mild epithelial necrosis and tubular dilation (C and D).
Figure 3. Haematoxylin and eosin stained sections of rat kidneys. (A) Normal kidney section. (B) Kidney section of a glycerol-treated rat showing severe tubular necrosis and cast formation. (C) Kidney section of a L-citrulline (900 mg/kg) + glycerol treated rat showing moderate necrosis and cast formation. (D) Kidney section of a dexamethasone + glycerol treated rat showing moderate necrosis and cast formation.
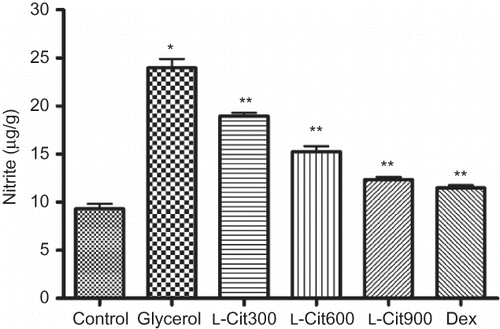
Concerning the effect of treatments on kidney nitrite (), L-citrulline decreased significantly kidney nitrite as compared to the rats that were only given glycerol, with non-significantly difference with the dexamethasone treated group. TNOS activity of the kidney tissues was found to be higher in the glycerol group compared to the normal control group (p < 0.01). Administrations of L-citrulline significantly decreased the TNOS compared with that of the glycerol group (p < 0.01). Additionally, the eNOS activity was lower in glycerol group compared to the normal control group (p < 0.05), However, iNOS activity was significantly higher (p < 0.01) in glycerol-treated rats than in normal control rats. L-Citrulline and dexamethasone treatment significantly lowered the iNOS activity (p < 0.01) and increased the activity of eNOS in kidney tissue ().
DISCUSSION
Glycerol-induced myoglobinuric ARF is an extensively employed model to study the pathophysiological mechanisms responsible for ARF.Citation23 The pathogenic mechanisms in this model contains an interaction of ischemic injury, tubular nephrotoxicity caused by myoglobin, and the renal action of cytokines released after rhabdomyolysis.Citation2 The hemoglobin and myoglobin releasing into systemic circulation bring about acidosis, dehydration, shock, or other conditions associated with reduced renal perfusion which may lead to both direct toxic and hemodynamic abnormalities resulting in ARF.Citation24 In our study, we found the administrations of glycerol induced a significant increase in kidney tissue MDA levels, and a significant decrease in kidney tissue SOD and GSH levels, as well as severe renal morphological impairment, showing that the existence of an oxidative stress in the renal tubule in this model. And administration of animals with L-citrulline significantly reduced the renal dysfunction and improved the alterations observed with glycerol injections.
The elevation of Cr and BUN are considered as significant markers of renal dysfunction in that the Cr level depends on the glomerular filtration rate (GFR). Renal dysfunction diminishes the ability to filter Cr and the rise in Cr level results. And the GFR is considered to have been halved when the Cr level twice over the normal value. Elevated BUN is correlated either with an increased protein catabolism or a more efficient conversion of ammonia to urea as a result of the increased synthesis of enzymes involved in urea production.Citation25 In the present study, the glycerol-administered rats which were treated with L-citrulline and dexamethasone had an significant decrease in the Cr and BUN levels compared with the model group which indicated that the improvement to the renal dysfunction.
In several studies, it has been demonstrated that ROS plays an important role in the pathogenesis of myoglobinuric ARF, the free oxygen radicals can cause lipid peroxidation of cellular membranes which leads to epithelial tubular cell necrosisCitation1,7–9 and lipid peroxidation was an important contributing factor to the development of kidney damage in the present study. However, we found that L-citrulline has an improvement in renal cell energy metabolism facilitates the repair of oxidized membrane/Lipid bilayers and the phenomenon of functional, biochemical, and morphological renal impairment which connected to the lipid peroxidation was prevented by the antioxidant effect. Also, L-citrulline activity as a direct ROS scavenger has been reported,Citation19 and it could indirectly increase in the intracellular GSH and SOD in the rat kidney tissues.
The data presented in this study demonstrated that the NO level in the group 2 is higher than the other groups suggesting that NO is an important mediator in this model. In kidney tissue, vascular endothelial cells in culture release NO which is one of the smallest biologically active messenger molecules and an important molecule in the regulation of renal microvascular tone, plays an important role in renal hemodynamics and in sodium and water tubular transport.Citation10,26,27 And many studies have clearly demonstrated that inappropriate high release of NO related to the tubular injury, it can disrupt mitochondrial respiration, damage membranes and DNA,Citation28–30 and also release iron from FeS complexes which can promote free radical formation lipid peroxidation, and renal dysfunction.Citation5,6 The overproduction of NO is mainly derived from iNOS, and the ROS is able to rapidly remove NO by its conversion into toxic peroxynitrite.Citation31 L-Citrulline treatment may lead to a reduction of NO inactivation by ROS with enhanced NO bioavailability. And our results support the beneficial role of L-citrulline as an antioxidant since it achieves lower level of NO in L-citrulline plus glycerol groups than the model group, at least in part, to the renoprotective effect of L-citrulline in the present study.
Dexamethasone is a corticosteroid which has long been proposed as a therapeutic adjuvant in renal dysfunction because of its anti-inflammatory properties and favorable results from animal experiments, particularly in the notion to correct a sepsis-related relative adrenal insufficiency. Previous study (Johannes et al.10) has indicated that the overproduction of NO is mainly derived from iNOS, leading to peroxynitrite-related tubular injury, and administration of low-dose dexamethasone in rats could inhibit iNOS production, decrease iNOS protein stability and repress the iNOS gene expression, so that the production of NO was inhibited. It has been demonstrated that the activity of iNOS is significantly higher in rats which only received glycerol than the normal control rats, which suggested that the iNOS activity might play a pivotal role in the glycerol-induced ARF. Moreover, the NO content was also higher in the model group than the other groups. However, the activity of iNOS and the NO content was significantly lowered in the rats receiving glycerol plus dexamethasone and L-citrulline, while the eNOS activity in kidney tissues was increased, which indicated that the L-citrulline might have the same protective effect as dexamethasone on relieving the glycerol-induced ARF.
In the current study, administrations of L-citrulline have a clearly beneficial effect on the severity of ARF. We have functionally, biochemically, and histopathologically demonstrated the protective effects of L-citrulline by reducing the severity of glycerol-induced myoglobinuric kidney damage. The effect of L-citrulline can be attributed to its reduction of oxidative damage, and its inhibitory effects on iNOS activity as well as upregulate eNOS activity in rat kidney tissues.
ACKNOWLEDGMENTS
The authors are cordially indebted to these financial supports: “Qing-Lan” Project of Jiangsu Province, the Industrialization of Scientific Research Promotion Projects of Universities and Colleges in Jiangsu Province (2011–2016), the Natural Science Fund for Universities and Colleges in Jiangsu Province (09KJB350003 and 11KJB350005), Laboratory of Biological Therapy for Cancer of Xuzhou Medical College (JSBL0803, C0903 and C0904), the Science and Technology Plan Projects of Xuzhou (XF11C037 and XF11C062), Superiority Academic Discipline Construction Project of Jiangsu Higher Education Institutions, and Xuzhou Public Service Platform Projects of Drug Discovery and Research, Innovation Project of Postgraduates in Jiangsu Province, China (CXLX11-0752).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Polo-Romero FJ, Fernández-Fúnez A, Broseta Viana L, Atienza MP, Sánchez Gascón F. Effect of N-acetylcysteine on antioxidant status in glycerol-induced acute renal failure in rats. Ren Fail. 2004;26:613–618.
- Chander V, Singh D, Chopra K. Attenuation of glycerol-induced acute renal failure in rats by trimetazidine and deferoxamine. Pharmacology. 2003;67:41–48.
- Abassi ZA, Hoffman A, Better OS. Acute renal failure complicating muscle crush injury. Semin Nephrol. 1998;18:558–565.
- Akpolat T, Akpolat I, Oztürk H, . Effect of vitamin E and pentoxifylline on glycerol-induced acute renal failure. Nephron. 2000;84:243–247.
- Zager RA. Rhabdomyolysis and myoglobinuric acute renal failure. Kidney Int. 1996;49:314–325.
- Rice-Evans C, Green E, Paganga G, Cooper C, Wrigglesworth J. Oxidised low density lipoproteins induce iron release from activated myoglobin. FEBS Lett. 1993;326:177–182.
- Ustundag S, Sen S, Yalcin O, Ciftci S, Demirkan B, Ture M. L-Carnitine ameliorates glycerol-induced myoglobinuric acute renal failure in rats. Renal Fail. 2009;31:124–133.
- Chander V, Chopra K. Molsidomine, a nitric oxide donor and L-arginine protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Biochim Biophys Acta. 2005;1723:208–214.
- Polo-Romero FJ, Fernández-Fúnez A, Broseta Viana L, Atienza MP, Sánchez Gascón F. Effect of N-acetylcysteine on antioxidant status in glycerol-induced acute renal failure in rats. Renal Fail. 2004;26:613–618.
- Johannes T, Mik EG, Klingel K, Dieterich HJ, Unertl KE, Ince C. Low-dose dexamethasone-supplemented fluid resuscitation reverses endotoxin-induced acute renal failure and prevents cortical microvascular hypoxia. Shock. 2009;31:521–528.
- Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61:855–861.
- Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci USA. 1990;87:10043–10047.
- Tsao CM, Ho ST, Chen A, . Low-dose dexamethasone ameliorates circulatory failure and renal dysfunction in conscious rats with endotoxemia. Shock. 2004;21:484–491.
- Leach M, Hamilton LC, Olbrich A, Wray GM, Thiemermann C. Effects of inhibitors of the activity of cyclo-oxygenase-2 on the hypotension and multiple organ dysfunction caused by endotoxin: a comparison with dexamethasone. Br J Pharmacol. 1998;124:586–592.
- Sureda A, Cordova A, Ferrer MD, . Effects of L-citrulline oral supplementation on polymorphonuclear neutrophils oxidative burst and nitric oxide production after exercise. Free Radic Res. 2009;43:828–835.
- Lau T, Owen W, Yu YM, . Arginine, citrulline, and nitric oxide metabolism in end-stage renal disease patients. J Clin Invest. 2000;105:1217–1225.
- Ochiai M, Hayashi T, Morita M, . Short-term effects of L-citrulline supplementation on arterial stiffness in middle-aged men. Int J Cardiol. 2010;155:257–261.
- Wu G, Collins JK, Perkins-Veazie P, . Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr. 2007;137:2680–2685.
- Liu Y, Tian X, Gou L, . Protective effect of L-citrulline against ethanol-induced gastric ulcer in rats. Environ Toxicol Pharmacol. 2012;34:280–287.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxide for animal tissues by thiobarbituric acid reaction. Anal Biochem. 1989;95:351–358.
- Ellman GL. Tissue sulfidryl group. Arch Biochem Biophys. 1959;82:70–77.
- Khattab MM, Gad MZ, Abdallah D. Protective role of nitric oxide in indomethacin-induced gastric ulceration by a mechanism independent of gastric acid secretion. Pharmacol Res. 2001;43:463–467.
- Reineck HJ, O’Connor GJ, Lifschitz MD, Stein JH. Sequential studies on the pathophysiology of glycerol-induced acute renal failure. J Lab Clin Med. 1980;96:357–362.
- Dubrow A, Flamenbaum W. Acute renal failure associated with myoglobinuria and hemoglobinuria. In: Solez K, Whelton A, eds. Acute Renal Failure. New York: Dekker; 1983:279–293.
- Rodwell EW. Catabolism of protein and of amino acid metabolism. In: Harper HA, Rodwell EW, Mayes P, eds. A Review of Physiological Chemistry. 17th ed. California: Lange Medical Publications; 1979:401–404.
- Yu L. Role of nitric oxide in acute renal failure. Renal Fail. 1997;19:213–216.
- Anbar M. Nitric oxide: a synchronizing chemical messenger. Experientia. 1995;51:545–550.
- Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995;369:136–139.
- Valdivielso JM, Blantz RC. Acute renal failure: is nitricoxide the bad guy? Antioxid Redox Signal. 2002;4:925–934.
- Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA. 1992;89:3030–3034.
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827.