Abstract
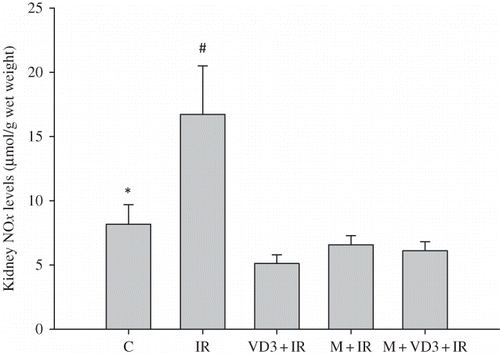
Ischemia–reperfusion (I/R) injury induces the generation of reactive oxygen species (ROS) which affect many organs. This study was designed to investigate the roles of melatonin and 1,25-dihydroxyvitamin D3 (VD3) on renal I/R injury. Thirty male Wistar albino rats were divided into five groups: group 1, control; group 2, right nephrectomy (RN) + I/R in the contralateral kidney; group 3, melatonin + RN + I/R; group 4, VD3 + RN + I/R; and group 5, melatonin + VD3 + RN + I/R. Melatonin (10 mg/kg), VD3 (0.5 μg/kg), and melatonin plus VD3 were injected intraperitoneally for 7 days before renal I/R. After 7 days, right nephrectomy was initially performed and left renal artery was clamped for 45 min. After 45-min reperfusion, the serum and kidney tissue samples were obtained for assays. Melatonin and VD3 had an ameliorative effect on biochemical parameters such as serum creatinine (SCr) and blood urea nitrogen (BUN). Renal tissue malondialdehyde (MDA), glutathione (GSH), nitric oxide (NO) levels, and superoxide dismutase (SOD) activity were determined. Renal I/R decreased the kidney tissue GSH levels and SOD activity and increased the NO levels as compared with control group. However, melatonin and VD3 and melatonin plus VD3 treatment significantly increased the tissue GSH levels and SOD activity and decreased the NO levels compared with those of I/R group. Meanwhile, MDA levels were not different between the control and I/R groups. But, MDA levels decreased in all treated groups compared to I/R and control groups. These data support that melatonin and VD3 have beneficial effects on renal injury.
INTRODUCTION
Cessation of kidney blood supply leads to acute renal failure (ARF), causing failure of the kidneys over a period of hours or days.Citation1 ARF has relatively high incidence rate in hospitalized patients and the most prevalent causes are ischemic events.Citation2 Ischemia–reperfusion (I/R) injury is a complex phenomenon and it leads to structural and functional cell damage.Citation3 One of the most important factors in pathophysiology of renal IR injury is reactive oxygen species (ROS), which especially increases in the reperfusion phase.Citation2 The endogenous antioxidants which are responsible for defence against ROS during reperfusion have an important role in decreasing IR injury.Citation2 ROS production is controlled by enzymatic and non-enzymatic processes.Citation4 Superoxide dismutase (SOD) and glutathione (GSH) are, respectively, one of the enzymatic and non-enzymatic antioxidants.Citation4 Oxidative stress is the usual phrase used to identify the association of the toxic free radicals with damage to cells and tissues.Citation5 There are controversial results of antioxidant and oxidant system status after I/R injury.
The pineal hormone melatonin is a potent free radical scavenger that protects the cells from damage induced by a variety of oxidants, including hydroxyl radical and lipid peroxidation products.Citation6,7 Vitamin D3 is taken in the diet or produced in the skin from 7-dehydrocholesterol in the skin by ultraviolet irradiation.Citation8 Vitamin D3 is transported in the circulation by the DBP (vitamin D binding protein).Citation9 Once delivered to the liver, vitamin D is hydroxylated on its side chain to form 25OHD. The kidney is the primary site where the active form of vitamin D, 1,25(OH)2D (VD3), is produced.Citation9 VD3 exhibits various physiological actions, including the regulation of calcium homeostasis, cellular differentiation and proliferation, immune functions, and reproduction.Citation10 VD3 has been shown to induce the expression of several enzymes involved in the antioxidant defence system.Citation9 There is also some evidence that VD3 regulates the genes for proteins that protect the genome.Citation9 Little information is available for the antioxidant property of VD3. In addition, there are controversial results between studies related to antioxidant properties of VD3.Citation11–14
Nitric oxide (NO) is synthesized from l-arginine by the enzyme nitric oxide synthase (NOS).Citation15 NO is produced by any of the three NO synthases (NOS). Endothelial (eNOS) and neuronal (nNOS) isoforms have been identified in the renal vasculature and macula densa, respectively.Citation16,17 The third inducible NOS (iNOS) is expressed in several segments of the renal tubule as well as in the glomerulus and interlobar and arcuate arteries of the normal rat kidney.Citation16,18 NO is recognized as an important mediator of physiological and pathological processes of renal I/R injury.Citation16
The aim of this study is to evaluate the changes in renal tissue antioxidant and oxidant status in I/R injury and whether melatonin and VD3 or melatonin plus VD3 treatment influence these parameters.
MATERIALS AND METHODS
Animals and Surgery
The experimental protocol was approved by Animal Ethics Committee of Maltepe University. Adult male Wistar rats, weighing approximately 200–250 g, were randomly divided into five groups of six rats in each group as follows: group 1, control; group 2, right nephrectomy (RN) + I/R in the contralateral kidney; group 3, melatonin + RN+ I/R; group 4, VD3 (1,25-dihydroxyvitamin D3) + RN + I/R; and group 5, melatonin + VD3 + RN + I/R. VD3 (Deva, Kocaeli/Turkey) was diluted with sterile saline and adjusted to a final concentration of 0.5 μg/kg. A week prior to I/R, VD3 was injected intraperitoneally for 7 days to the VD3 + I/R group. Melatonin 10 mg/kg, (M5250 Sigma Chemical, St Louis, MI, USA) was injected intraperitoneally for 7 days to the melatonin +I/R group, and melatonin 10 mg/kg + VD3 0.5 μg/kg was injected to the melatonin + VD3 + I/R group. Following a 12-h fasting period, control, VD3, melatonin, VD3 + melatonin groups were anesthetized with intramuscular ketamine 50 mg/kg and xylazine 10 mg/kg.
All rats had right nephrectomy, with a dorsal flank incision. In the I/R groups, the left renal pedicle is clamped with a non-crushing microvascular clamp (Bulldog Artery Clamp, Harvard Apparatus, MA, USA) for 45 min and the presence of ischemia is visually confirmed by observing blanching of the kidney. Forty-five minutes of reperfusion period followed the ischemia period. At the end of the time, blood was collected by heart puncture for measurement of biochemical analysis and the kidney was removed for GSH, SOD, malondialdehyde (MDA), and NO measurements.
BUN and Creatinine Assay
Serum samples were used for the measurement of blood urea nitrogen (BUN) and serum creatinine (SCr) levels, which were used as indicators of impaired glomerular function. BUN and SCr were determined with an Abbott-Aeroset autoanalyzer (Chicago, IL, USA) using original kits.
MDA and GSH Assay
Lipid peroxidation was quantified by measuring the formation of thiobarbituric acid reactive substances as described previously.Citation19 Lipid peroxidation levels were expressed in terms of MDA equivalents using an extinction coefficient of 1.56 × 105/mol/cm. Tissue samples were homogenized in ice-cold trichloroacetic acid (1 g tissue plus 10 ml 10% trichloroacetic acid). The GSH level was determined by a modified Ellman method.Citation20 Briefly after centrifugation at 3000 rpm for 10 min, the supernatant was collected. Two milliliter of 0.3 M Na2HPO4·2H2O solution was added to 0.5 ml of the supernatant. Afterwards, a 0.25 ml solution of dithiobisnitrobenzoate (0.4 mg/mL in 1% sodium citrate) was added to this mixture and then mixture was vortexed. After 10 min incubation in room temperature, absorbances of the samples were measured at 412 nm spectrophotometrically. The GSH levels were calculated using an extinction coefficient of 13,000 mol/cm.
Superoxide Dismutase Assay
The SOD assay kit is used for the measurement of SOD activity in the kidney (Cayman Chemical Co., Ann Arbor, MI, USA). SOD activity is assessed by measuring the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine.
Determining the Tissue Total NO(NOX) Levels
The NOx levels were determined by vanadium chloride (VCl3)/Griess assay. Prior to NOx determination, the tissues were homogenized in five volumes of phosphate-buffered saline (pH = 7.5) and centrifuged at 2000 × g for 5 min. Then, 0.25 ml of 0.3 M NaOH was added to 0.5 ml supernatant. After incubation for 5 min at room temperature, 0.25 ml 5% (w/v) ZnSO4 was added for deproteinization. This mixture was then centrifuged at 3000× g for 20 min and the supernatants were used for the assays as described previously.Citation21,22
Light Microscopy
Renal tissue samples were fixed in 10% neutral-buffered formalin, followed by embedding in paraffin wax, cut into 4-μm sections, and stained with periodic acid Schiff (PAS). Evaluation was performed with light microscopy without knowledge of the study groups. Assessment was carried out by expert observers.
Statistical Analyses
All data are expressed as mean ± standard error of the mean (SEM). The analyses were performed using SigmaStat 3.5 (Systat Software, Inc., Richmond, CA, USA). Differences in measured parameters among the groups were analyzed by Kruskal–Wallis test. Dual comparisons between groups that present significant values were evaluated with Mann–Whitney U test. Statistical significance was accepted at a p-value of < 0.05.
RESULTS
There was a significant increase in the SCr and BUN levels in the I/R group compared to the control rats (p = 0.004). Administration of melatonin, VD3, and melatonin + VD3 significantly decreased the SCr and BUN levels compared to I/R group ( and ).
Figure 1. Creatinine levels. Results are expressed as a mean ± SEM.Note: *C versus IR and C versus VD3 p = 0.004; #IR versus VD3, p = 0.009; IR versus M and IR versus M + VD3, p = 0.004. C: control, I/R: ischemia–reperfusion, VD3: 1,25-dihydroxyvitamin D3, M: melatonin, M + VD3: melatonin + 1,25-dihydroxyvitamin D3.
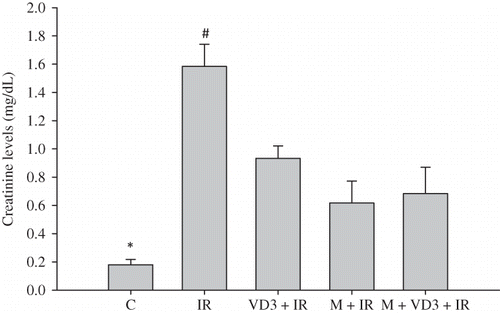
Figure 2. Blood urea nitrogen (BUN) levels.Note: *C versus IR and C versus VD3, p = 0.004; #IR versus VD3, IR versus M and IR versus M + VD3, p = 0.002. For abbreviations, see legend to Figure 1.
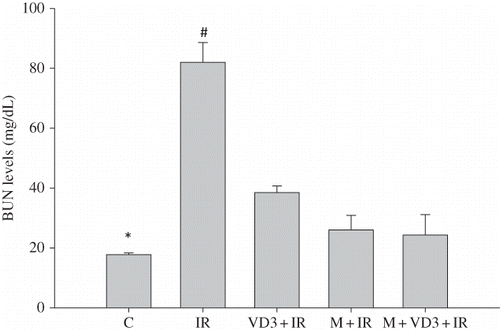
Renal I/R caused a marked decrease in kidney tissue GSH levels as compared to control group (p = 0.015). However, melatonin and VD3 and melatonin plus VD3 treatment significantly increased tissue GSH levels compared with those of I/R group ().
Figure 3. Renal tissue glutathione (GSH) levels.Note: *C versus IR and p = 0.015; #IR versus VD3 and IR versus M, p = 0.009. For abbreviations, see legend to Figure 1.
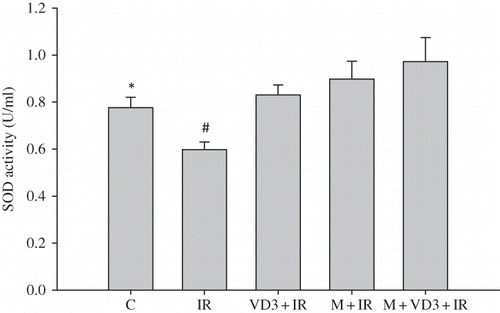
Kidney SOD activity was decreased by renal I/R as compared to control group (p = 0.016). The VD3, melatonin, and melatonin plus VD3-treated rat kidneys showed a significantly increased SOD activity as compared with I/R group without treatments ().
Figure 4. Renal tissue superoxide dismutase (SOD) activities.Note: *C versus IR, p = 0.016; #IR versus VD3 and IR versus M, p = 0.004; IR versus M + VD3, p = 0.03. For abbreviations, see legend to Figure 1.
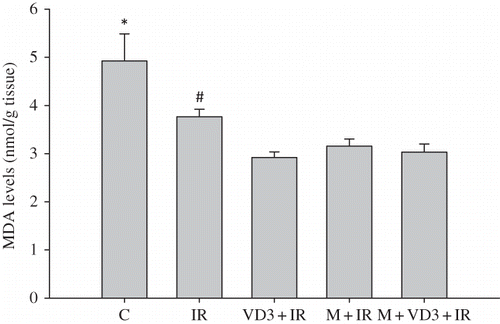
Treatment with melatonin, VD3, and melatonin plus VD3 significantly reduced the tissue MDA levels as compared to I/R group without treatment (). On the other hand, MDA levels were not statistically significant between I/R and control groups.
Figure 5. Renal tissue malondialdehyde (MDA) levels.Note: *C versus VD3, p = 0.002; C versus M and C versus M + VD3, p = 0.004; #IR versus VD3, p = 0.009; IR versus M and IR versus M +VD3, p = 0.015. For abbreviations, see legend to Figure 1.
Kidney NOx levels were increased in the I/R group compared to the control group (p = 0.015). NO levels were found to be significantly lower in all treatment groups compared to the I/R group ().
HISTOPATHOLOGIC RESULTS (PAS)
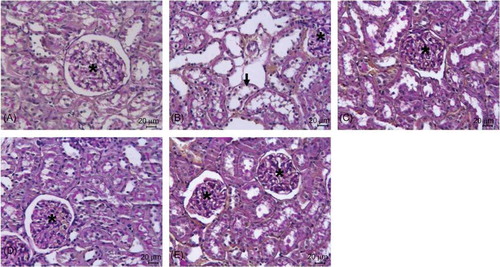
Renal morphology was normal in control group (A). Extensive necrosis plus dilation, flattening of epithelium, and loss of brush border in proximal tubuli (arrow) were observed in the I/R group (B). In Mel + I/R (C), VD3+ I/R (D) and Mel + VD3+ I/R (E) groups, better histopathologic findings compared to the I/R group were observed.
Figure 7. PAS staining of kidney sections of control (A), I/R (B), Mel + I/R (C), VD3+ I/R (D), and Mel + VD3+ I/R (E) groups. In the I/R group, extensive necrosis plus dilation, flattening of epithelium, and loss of brush border in proximal tubuli (arrow) were seen (Glomeruli; asterisk) (bar = 20 μm).
DISCUSSION
Renal ischemia is a major cause of acute renal failure, which despite significant advances in critical care medicine remains a major clinical problem, producing grave morbidity and mortality that has not decreased significantly over the last 50 years.Citation23 Chronic kidney disease (CKD) has been shown to result in decreased vitamin D metabolism through multiple mechanisms.Citation8,24 The loss of functional renal mass in CKD, cause of decreased production of 1α(OH)ase, leads to decreased VD3 levels.Citation8,24 In the present study, in order to evaluate the biochemical parameters within the reversible first phase of reperfusion injury extending only for a few hours, the reperfusion interval is limited to 45 min. In our study, renal I/R increased serum BUN and SCr levels. Treatment with melatonin and VD3 or melatonin plus VD3 prior to renal I/R significantly reduced the serum levels of BUN and SCr. Significant increases in the serum concentrations of BUN and Cr subsequent to I/R suggest the impairment of glomerular function.Citation25,26 I/R of the kidney causes both glomerular and tubular dysfunction.Citation25 It was reported that VD3 treatment may accelerate the recovery of renal tubular cells by increasing the proliferation of renal tubular cells in ischemic rat kidneys.Citation27 In this study, 0.5 μg/kg/day of VD3 was used, tolerable dose, based on the Kim et al.Citation27 research.
Reperfusion of the kidneys causes the activation and adhesion of polymorphonuclear neutrophils (PMNLs), with the release of proinflammatory substances and the formation of free radicals, which are nitrogen-derived reactive nitrogen species (RNS) or oxygen-derived reactive oxygen species (ROS) such as superoxide anion (O2●[?tf][?tvs][?down]?>•−), hydrogen peroxide (H2O2), and hydroxyl radicals (●[?tf][?tvs][?down]?>•OH).Citation1,28–30 Recognizing IR effects on antioxidant enzymes activities and GSH level may help to find a better strategy for prevention and/or therapy of ischemic ARF.Citation2 SOD is the first line of defence of the cellular antioxidant system against the oxidative damage mediated by superoxide radicals.Citation13 It was reported that VD3 induces the expression of several antioxidant enzymes.Citation9 In addition, mRNA levels for the essential antioxidant enzymes SOD1 and SOD2 are induced by VD3 in primary PECsCitation9,31 and LNCaP cells,Citation9,32 respectively.
There is little information for the antioxidant property of VD3 and there are controversial results between studies related to antioxidant properties of VD3.Citation11,13,14 There are many studies concerning the antioxidant properties of melatonin.Citation7,33,34 It was also reported that melatonin has a protective effects on kidney tissue against oxidative damage and apoptosis.Citation35,36 But for the first time, this study evaluated the combined use of melatonin and VD3 against renal I/R injury. Most previous studies have been shown to decrease in SOD and catalase activities and GSH level after IR.Citation14,23,29,37,38 However, Rasoulian et al.2 showed a decrease in renal GSH level, increase in renal SOD activity, and unchanged MDA levels following IR. On the other hand, Ersoz et al.Citation1 found that SOD activity and MDA levels significantly increased after IR. Ekici et al.Citation14 reported that VD3 treatment showed no significant effect on decreased SOD activity and GSH levels and increased MDA levels caused by cerebral IR. However, the combination of VD3 and Vitamin C treatment showed a significant decrease in MDA levels and a significant increase in SOD activity and GSH levels.Citation14 The results of our present investigation demonstrated that SOD activity and GSH levels decreased after IR compared with the control group. But, MDA levels did not change after IR. However, treatment with melatonin and VD3 or melatonin plus VD3 prior to renal I/R increased the SOD activity and GSH levels. We suggest that pretreatment with VD3 and melatonin or the combination of VD3 and melatonin have a protective effect on renal I/R injury by increasing SOD activity and GSH levels.
It is now widely accepted that the NO pathway is involved in the functional regulation of any organ, tissue, and cell in the body.Citation39 Various studies have shown that NO biosynthesis and its action are closely related to the pathogenesis of renal I/R injury.Citation1,2,16,23 NO is produced by the endothelial cells and causes relaxation of preglomerular arteries improving renal blood flow.Citation40 During renal I/R, ROS can disrupt the integrity of the endothelium and can affect NO production, resulting in increment of renal vascular resistance.Citation40 Chander and ChopraCitation23 reported that kidney NO levels decreased in glycerol-induced acute renal failure in rats. On the other hand, some studies showed a significant increase in NO levels after renal I/R injury.Citation1,16,38 In our study, renal I/R injury increased NO levels in kidney tissue. Our data are in agreement with these studies,Citation1,16,38 but contrary to the observation of Chander and Chopra.Citation23 Treatment with VD3 and melatonin alone or the combination of these substances caused a significant decrease in renal NO levels. Ersoz et al.Citation1 reported that NO-induced I/R injury may be a result of the development of ONOO−, as NO couples with O2−. iNOS plays a deleterious role during renal I/R injury. Melatonin has the ability to scavenge many of the oxygen-based and nitrogen-based radicals, including ONOO−. It was suggested that NOS activity, probably endothelial constitutive NOS (cNOS), increased in response to ischemia-induced renal vasoconstrictor activity.Citation41 Ischemic injury itself may increase smooth muscle cytosolic calcium or increase oxygen radical generation, providing potent vasoconstrictor stimulation to which NOS activation is a modulating response.Citation41 Increased NO levels in I/R injury may be due to increased NOS activity and ROS/RNS production. It has been shown that VD3Citation42,43 and melatoninCitation44,45 inhibit expression of NOS. In our study, NOS was not evaluated. Treatment with these substances prior to I/R injury could decrease NOS activity and ROS/RNS formation. For these reasons, pre-ischemic treatment with these substances may reduce NO levels.
In summary, the maintaining of balance between oxidant and antioxidant system in preventing I/R injury may be an important therapeutic strategy. In other words, increase in renal antioxidant enzymes and decrease in renal production of ROS may help to prevent I/R injury. Treatment with melatonin and VD3 or melatonin combination with VD3 have been shown to increase in renal SOD activity and GSH levels and decrease in NOx levels. There are many studies for melatonin to prevent I/R damage, but little information for using VD3. Our data suggest that VD3 participates in the antioxidant system to protect the renal tissue against I/R injury. Therefore, VD3 should be further evaluated for use in I/R injury.
Declaration of interest: The authors report no conflicts of interest.
This study was funded by Maltepe University (2011-04).
REFERENCES
- Ersoz N, Guven A, Cayci T, Comparison of the efficacy of melatonin and 1400W on renal ischemia/reperfusion injury: a role for inhibiting iNOS. Ren Fail. 2009;31(8):704–710.
- Rasoulian B, Jafari M, Noroozzadeh A, Effects of ischemia-reperfusion on rat renal tissue antioxidant systems and lipid peroxidation. Acta Medica Iranica. 2008;46:353–360.
- Rodriguez-Reynoso S, Leal C, Portilla-de BE, Castillo JC, Ramos-Solano F. Melatonin ameliorates renal ischemia/reperfusion injury. J Surg Res. 2004;116(2):242–247.
- Takahashi M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev. 2012; 1–9.
- Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995;9(7):526–533.
- Bonnefont-Rousselot D, Collin F, Jore D, Gardes-Albert M. Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. J Pineal Res. 2011;50(3):328–335.
- Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51(1):1–16.
- Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin North Am. 2010;39(2):243–253.
- Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441(1):61–76.
- Khan KA, Akram J, Fazal M. Hormonal actions of vitamin D and its role beyond just being a vitamin: a review article. Int J Med Med Sci. 2011;3:65–72.
- Lee J, Youn JI. The photoprotective effect of 1,25-dihydroxyvitamin D3 on ultraviolet light B-induced damage in keratinocyte and its mechanism of action. J Dermatol Sci. 1998;18(1):11–18.
- Noyan T, Balaharoglu R, Komuroglu U. The oxidant and antioxidant effects of 25-hydroxyvitamin D3 in liver, kidney and heart tissues of diabetic rats. Clin Exp Med. 2005;5(1):31–36.
- Banakar MC, Paramasivan SK, Chattopadhyay MB, 1alpha, 25-dihydroxyvitamin D3 prevents DNA damage and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World J Gastroenterol. 2004;10(9):1268–1275.
- Ekici F, Ozyurt B, Erdogan H. The combination of vitamin D3 and dehydroascorbic acid administration attenuates brain damage in focal ischemia. Neurol Sci. 2009;30(3):207–212.
- Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43(3):521–531.
- Vinas JL, Sola A, Genesca M, Alfaro V, Pi F, Hotter G. NO and NOS isoforms in the development of apoptosis in renal ischemia/reperfusion. Free Radic Biol Med. 2006;40(6):992–1003.
- Kone BC. Nitric oxide in renal health and disease. Am J Kidney Dis. 1997;30(3):311–333.
- Mattson DL, Wu F. Nitric oxide synthase activity and isoforms in rat renal vasculature. Hypertension. 2000;35(1 Pt 2):337–341.
- Casini AF, Ferrali M, Pompella A, Maellaro E, Comporti M. Lipid peroxidation and cellular damage in extrahepatic tissues of bromobenzene-intoxicated mice. Am J Pathol. 1986;123(3):520–531.
- Aykac G, Uysal M, Yalcin AS, Kocak-Toker N, Sivas A, Oz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology. 1985;36(1):71–76.
- Taskiran D, Kutay FZ, Sozmen E, Pogun S. Sex differences in nitrite/nitrate levels and antioxidant defense in rat brain. NeuroReport. 1997;8(4):881–884.
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71.
- Chander V, Chopra K. Protective effect of resveratrol, a polyphenolic phytoalexin on glycerol-induced acute renal failure in rat kidney. Ren Fail. 2006;28(2):161–169.
- Cheng S, Coyne D. Vitamin D and outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2007;16(2):77–82.
- Chatterjee PK, Brown PA, Cuzzocrea S, Calpain inhibitor-1 reduces renal ischemia/reperfusion injury in the rat. Kidney Int. 2001;59(6):2073–2083.
- Stogdale L. Correlation of changes in blood chemistry with pathological changes in the animal’s body: II Electrolytes, kidney function tests, serum enzymes, and liver function tests. J S Afr Vet Assoc. 1981;52(2):155–164.
- Kim YO, Li C, Sun BK, Preconditioning with 1,25-dihydroxyvitamin D3 protects against subsequent ischemia-reperfusion injury in the rat kidney. Nephron Exp Nephrol. 2005;100(2):e85–e94.
- Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109(8):665–678.
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282(2):C227–C241.
- Greene EL, Paller MS. Xanthine oxidase produces O2−. in posthypoxic injury of renal epithelial cells. Am J Physiol. 1992;263(2 Pt 2):F251–F255.
- Peehl DM, Shinghal R, Nonn L, Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem Mol Biol. 2004;92(3):131–141.
- Lambert JR, Kelly JA, Shim M, Prostate derived factor in human prostate cancer cells: Gene induction by vitamin D via a p53-dependent mechanism and inhibition of prostate cancer cell growth. J Cell Physiol. 2006;208(3):566–574.
- Ozturk G, Coskun S, Erbas D, Hasanoglu E. The effect of melatonin on liver superoxide dismutase activity, serum nitrate and thyroid hormone levels. Jpn J Physiol. 2000;50(1):149–153.
- Rodriguez C, Mayo JC, Sainz RM, Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9.
- Sener G, Sehirli AO, Keyer-Uysal M, Arbak S, Ersoy Y, Yegen BC. The protective effect of melatonin on renal ischemia-reperfusion injury in the rat. J Pineal Res. 2002;32(2):120–126.
- Sinanoglu O, Sezgin G, Ozturk G, Melatonin with 1,25-Dihydroxyvitamin D3 Protects against Apoptotic Ischemia-Reperfusion Injury in the Rat Kidney. Ren Fail. 2012;34(8):1021–1026.
- Wang F, Yu G, Liu SY, Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res. 2011;167(2):e339–e344.
- Vaghasiya J, Sheth N, Bhalodia Y, Shailesh M, Jivani N. Exenatide protects renal ischemia reperfusion injury in type 2 diabetes mellitus. Int J Diab Dev Ctries. 2010;30:217–225.
- Muriel P. Regulation of nitric oxide synthesis in the liver. J Appl Toxicol. 2000;20(3):189–195.
- Rhoden EL, Rhoden CR, Lucas ML, Pereira-Lima L, Zettler C, Bello-Klein A. The role of nitric oxide pathway in the renal ischemia-reperfusion injury in rats. Transpl Immunol. 2002;10(4):277–284.
- Conger J, Robinette J, Villar A, Raij L, Shultz P. Increased nitric oxide synthase activity despite lack of response to endothelium-dependent vasodilators in postischemic acute renal failure in rats. J Clin Invest. 1995;96(1):631–638.
- Garcion E, Sindji L, Leblondel G, Brachet P, Darcy F. 1,25-dihydroxyvitamin D3 regulates the synthesis of gamma-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J Neurochem. 1999;73(2):859–866.
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105.
- Bettahi I, Pozo D, Osuna C, Reiter RJ, Acuna-Castroviejo D, Guerrero JM. Melatonin reduces nitric oxide synthase activity in rat hypothalamus. J Pineal Res. 1996;20(4):205–210.
- Esposito E, Iacono A, Muia C, Signal transduction pathways involved in protective effects of melatonin in C6 glioma cells. J Pineal Res. 2008;44(1):78–87.