Abstract
Background: Erythropoietin (EPO) formulations may comprise aluminum (Al) as a contaminant. Due to the toxicity of Al in chronic kidney disease patients, possible sources of Al were investigated. Since EPO formulations are stored in container-closure systems made of glass and rubber, and both contain Al, formulation ingredients may enable its leaching into the solution during shelf-life. Methods: Individual solutions of formulation ingredients were stored in new glass vials and in contact with the rubber stopper and kept at 4 ± 2°C. For 12 months, aliquots of each solution were collected for analysis. Fifteen commercial samples of EPO were analyzed for their Al content. Aluminum was determined by atomic absorption spectrometry. Results: Glass and rubber are sources of Al for EPO formulations. Storage assay showed that citrate and phosphate (used as buffers) extracted high amounts of Al from the container/closure parts. The most important difference, however, was found when comparing liquid and lyophilized samples. While in liquid forms the Al level reached 943 μg/L, in lyophilized forms the level did not exceed 20 μg/L. The container system was also confirmed as a source of Al in reconstituted lyophilized samples. Al in reconstituted samples stored in their own vials increased 19-fold in 12 months. Lyophilized powders stored for 2 years in glass vials contained less Al than in 1 month after dissolution. Conclusion: The difference in the Al measured in liquid forms of EPO and in lyophilized powders suggests that the latter would be the best pharmaceutical form for CKD patients.
INTRODUCTION
The introduction of recombinant human erythropoietin in the early 1990s provided the first effective treatment for the anemia of chronic kidney disease (CKD). Currently, more than 90% of patients on regular dialysis therapy worldwide receive erythropoietin.Citation1
Pharmaceutical products often contain agents other than the active ingredient that are added for a variety of purposes. Frequently, these additives make up the majority of the mass or volume of a drug product, which is the case of EPO formulations.
Commercial EPO formulations are supplied as a solution or as a lyophilized powder, and dosages from 1.000 to 40.000 IU of EPO are available. In addition to EPO, formulations contain inactive ingredients (human serum albumin, glycine, sodium citrate, and polysorbate 80, among others) to stabilize and buffer the preparate. All formulations—both solution and lyophilized powder—are supplied in pre-filled syringes or vials made of glass.
Besides the glass part, vials and syringes contain a rubber part: the vial stopper and the syringe plunger. Both glass and rubber contain aluminum in their constitution. Glass containers for parenteral products must meet requirements of chemical stability,Citation2,3 which are achieved by adding boric and aluminum oxides to common glass. As a result, glass for parenterals typically contains between 2.6% and 6.6% Al2O3.Citation4 On the other hand, aluminum silicate (clay) is used as a filler in elastomeric materials to improve hardness, abrasion resistance, and density.Citation5 The Al content in rubber for medicinal purposes may reach 6%.
This study investigated raw materials and container-closure systems as sources of Al in EPO formulations.
MATERIALS AND METHODS
Apparatus
Al measurements were performed using an Analytik Jena ZEEnit 600 atomic absorption spectrometer (Jena, Germany) equipped with a transversal heated graphite furnace, a Zeeman effect background corrector, and an autosampler. Other equipments that were employed include a Trox Class 100 clean bench (Curitiba, Brazil), an Ehret oven (Darmstadt, Germany), a Digimed pH meter D-20 (São Paulo, Brazil), and a Berghof BSB 939-IR sub-boiling distillation apparatus (Eingen, Germany).
Reagents
Albumin and EPO were purchased from Sigma-Aldrich (St Louis, MO, USA).
The assayed chemicals were the excipients used in the EPO formulations: sodium chloride (NaCl), sodium citrate, citric acid, disodium phosphate, sodium dihydrogenphosphate, calcium chloride (CaCl2), urea, mannitol, polysorbate 80, polysorbate 20, glycine, phenylalanine, leucine, isoleucine, threonine, and glutamic acid. These chemicals were of pharmaceutical or analytical grade and were purchased from different manufacturers (Merck, Aldrich, Sigma, Synth, and Fluka).
The water used throughout the investigation was distilled, deionized, and further purified by a Milli-Q high purity water device (Millipore, Bedford, MA, USA). An Al standard solution containing 1000 mg/L Al (SepSol, SEM 3101, Gaithersburg, MD, NIST-USA) was used to prepare standard solutions. HNO3 (65%, 1.40 g/mL) from Merck was further purified by sub-boiling distillation.
Contamination Control
To avoid contamination, only plastic materials were used. All laboratory equipments (pipette tips, volumetric flasks, etc.) were immersed for at least 48 h in a 10% (v/v) HNO3/ethanol solution and, shortly before use, washed with Milli-Q purified water. To avoid contamination from the air, all steps in the sample and reagent preparation were carried out in a Class 100 clean bench.
Procedures
The containers used in the experiments were new 50-mL glass vials with gray rubber stoppers for parenteral formulations (Schott, Rio de Janeiro, Brazil).
Solutions were prepared in polyethylene volumetric flasks, and the pH was adjusted to 7.3–7.4 with 0.1 mol/L HCl or NaOH solution accordingly. The Al content of these solutions before testing was measured by graphite furnace atomic absorption spectrometry (GF AAS). To allow a comparison among the different ingredients, solutions were prepared at a concentration of 0.05 mol/L.
Fifty milliliters of each individual solution was stored in a glass vial. Rubber stoppers were immersed in the same volume of solution, but in 50-ml conical plastic flasks. In both cases, flasks were closed and heated at 70°C for 30 min, and this process was repeated three times. After cooling, the Al content of the solutions was measured by GF AAS. These experiments were carried out in duplicate. In order to have a blank or control sample, purified water (pH adjusted to 7.3–7.4) was assayed along with the samples.
Although samples were heated to perform an accelerated aging test,Citation6 they were additionally stored for 12 months under refrigeration (4 ± 2°C), and aliquots were collected (after 1, 3, 5, 7, 9, and 12 months) for Al measurement.
Analysis of Commercial Erythropoietin Formulations
Fifteen different commercial samples of EPO were analyzed to determine the level of contamination by Al. Three samples of the same lot were analyzed, and the results correspond to the average of these measurements. Samples were diluted with water, and the Al content was measured by GF AAS. For lyophilized samples, the powder was reconstituted with purified water, and the water for injection (WFI), supplied for powder reconstitution, was analyzed separately. Reconstituted lyophilized samples were stored in their own vials and stored under refrigeration for 12 months. Aluminum was periodically measured in these samples.
RESULTS
The results of the analysis of commercial EPO commercial formulations are shown in . The measured Al levels confirm that EPO may be a significant source of Al for CKD patients.Citation7,8 Comparing different lots of the same formulation, it is possible to see that older vials possess higher Al contamination. On the other hand, there is a significant difference among the Al level in liquid and lyophilized forms. While in lyophilized samples a mean of 20 μg/L Al was found, in liquid samples this value was 381 μg/L Al.
Table 1. Aluminum contamination in commercial formulations of EPO.
After reconstitution of lyophilized powders as recommended by suppliers (but using Milli Q purified water), they were kept in the glass vial at 4°C for 12 months. The measurement of Al in these samples showed that the amount of Al in solution increased 19 times relative to the powder contamination levels ().
Figure 1. Amount of aluminum measured in lyophilized EPO samples after reconstitution and storage under refrigeration (4°C) for a period of 12 months. (•) Sample 13; (♦) sample 14; (▪) sample 15 in Table 1.
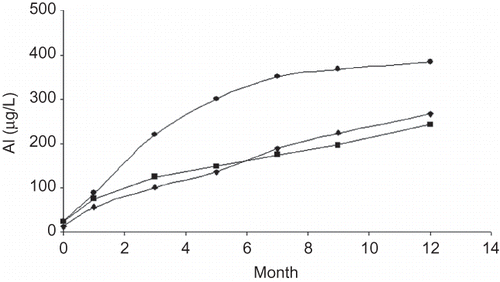
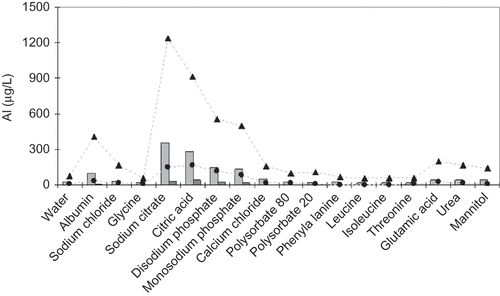
Figure 2. Amount of aluminum extracted from glass vials and rubber stoppers by contact with pure water or with solutions of the constituents of EPO formulations. Bars: after heating cycles (three times at 70°C) and after 12 months storage under refrigeration (4°C): (▴) storage in glass vials; (•) storage in contact with rubber stoppers.
shows the Al levels found in the components present in EPO formulations, including pure EPO. These data show that the raw materials themselves are unlikely to be responsible for the elevated levels of Al found in commercial samples. Although pure EPO is highly contaminated, the final solution contained only 5.6 μg/L Al.
Table 2. Levels of aluminum in the ingredients typically present in EPO formulations.
These results indicate that the container-closure system may be the main source of Al, and furthermore, that the migration is facilitated by the contact between the liquid formulation and the container.
Although glass and rubber contain Al, it would not migrate into the solution unless promoted by an external agent. In an earlier study, we showed that substances with affinity for Al were able to extract Al from the glass surface, and the higher the affinity, the greater the amount extracted.Citation9
The evaluation of the ingredients’ ability to promote the release of Al from glass and rubber showed that, although this occurred even in pure water, citrates, phosphates, and HSA extracted the highest amounts of Al. The other ingredients extracted as much Al as the water did. The interaction of the solutions with glass vials was higher than that with the rubber, probably due to the smaller solution/surface contact in the latter ().
Although stored under refrigeration, samples showed an increase in the Al concentration during the 12-month storage period. Again, citric acid, sodium citrate, phosphates, and HSA solutions were responsible for higher extraction yields.
An elevated amount of Al was measured in the WFI samples furnished with the lyophilized powder samples, ranging from 280.8 ± 2.5 to 334.8 ± 5.4 μg/L. The contamination of WFI for EPO reconstitution was already shown by Fleming et al.Citation10 In 36 ampoules of WFI supplied for diluting recombinant EPO, the measured Al content ranged from 24 to 450 μg/L. In their study, in EPO samples supplied already in solution, Al ranged from 506 to 837 μg/L. This elevated contamination of the WFI is originated from the sterilization procedure where sealed glass ampoules are heated at 121°C for 15–30 min. The heating causes Al to leach from the glass surface. By contrast, the level of Al in WFI stored in plastic containers is lower than 4 μg/L.Citation11
DISCUSSION
Citrate and phosphate are strong ligands for Al. The stability constants (calculated at pH 7.4) are 12.7 and 6.2, respectively.Citation12 The Al binding ability of citrate and phosphate is well known in nephrology. When aluminum hydroxide is used as a phosphate binder to control hyperphosphatemia among dialysis patients, citrate sources should be avoided because citrate enhances aluminum absorption.Citation13
The different buffer systems are probably responsible for the different amounts of Al found in the commercial samples (). While citrate/citric acid is the buffer used in the Alfapoetina samples, the Eritromax samples are buffered with phosphates. Samples of the same age present different levels of contamination: the amount of Al in Alfapoetina samples is approximately two times higher than the amount of Al in Eritromax samples.
Problems encountered with inactive ingredients are usually related to adverse reactions caused by the ingredient itself. Recently, an inactive ingredient of an EPO formulation, polysorbate 80, was suspected of promoting the leaching of compounds from the rubber syringe stoppers which caused antibody-mediated pure red cell aplasia in patients with CKD.Citation14 In this case, a minor ingredient was able to leach, from a small part of the container system, an amount of substances sufficient to initiate an immune response. In the case of Al, the risk is equally significant; however, due to the long-lasting deposition of Al in the bones, the body’s response is not immediate, and therefore no alert is produced.
The administration of contaminated EPO for an adult receiving 4000 IU EPO three times weekly, would supply 1.31 μg Al/week (considering the mean of liquid formulations in ) or 0.06 μg Al/week (considering the mean of lyophilized formulations in ). Taking into account the way of administration (subcutaneous or intravenous), the poor renal elimination, and the ineffective removal of Al from the circulation during dialysis due to its highly protein-bound nature, the difference between the types of samples (22 times) can account for Al accumulation. Assuming that the dietary daily intake ranges from 1.6 to 13 mg Al and the oral bioavailability in humans is 0.1% for food and beverages,Citation15 the amount of Al absorbed weekly from food would range from 0.48 to 39 μg, making 3.36–273 μg Al weekly. The intake through liquid form EPO would account for one-third of the lowest amount weekly absorbed via gastrointestinal.
CONCLUSION
Pharmaceuticals are stored in container systems made of glass and rubber which contain Al as a constituent. Ingredients in the EPO formulations are able to extract Al from glass and rubber surfaces during storage, and the longer the contact the higher, the degree of Al contamination. In addition, ingredients with affinity for Al caused larger amounts of Al extraction. EPO in liquid form presents much higher Al contamination than the lyophilized powder form. Therefore, lyophilized powder and the WFI for reconstitution supplied in plastic containers should be the form of choice for CKD patients.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENT
The authors are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasília, Brazil) for scholarships. The authors are grateful to the nursery staff of the Hospital de Caridade de Santa Maria and the University Hospital for the samples.
REFERENCES
- Pisoni RL, Bragg-Gresham JL, Young EW, Anemia management and outcomes from 12 countries in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2004;44(1):94–111.
- United States Pharmacopeia, USP 32; 2008.
- The Japanese Pharmacopoeia, Fifteenth Edition; 2009.
- Shelby JE. Introduction to Glass Science and Technology. Cambridge: The Royal Society of Chemistry; 1997.
- Smith EJ, Nash RJ. Elastomeric closures for parenterals. In: Avis KE, Lachman, L, Lieberman HA, eds. Pharmaceutical Dosage Forms: Parenteral Medications. Vol 2. New York: Marcel Dekker Inc; 1986:155–215.
- Waterman KC, Adami RC. Accelerated aging: prediction of chemical stability of pharmaceuticals. Int J Pharm. 2005;293:101–125.
- Bohrer D, Bertagnolli DC, de Oliveira SMR, do Nascimento PC, de Carvalho LM, Pomblum SG. Drugs as a hidden source of aluminium for chronic renal patientsNephrol Dial Transpl. 2007;22(2):605–611.
- de Carvalho LM, Garmatz JC, Spengler C, Determination of metallic contaminants in human recombinant erythropoietin (EPO) formulations by adsorptive cathodic stripping voltammetry and UV photo-decomposition of samples. J Braz Chem Soc. 2010;21(4):701–709.
- Bohrer D, do Nascimento PC, Martins P, Binotto R. Availability of aluminum from glass and an Al-form ion exchanger in the presence of complexing agents and amino acids. Anal Chim Acta. 2002;459:267–276.
- Fleming LW, Halls DJ, Henderson IS, Mactier RA, Taylor JE, Stewart WK. Aluminium content of water for injection used with recombinant human erythropoietin. Nephrol Dial Transpl. 1991;6(11):857–861.
- Bohrer D, do Nascimento PC, Binotto R, Becker E. Influence of the glass packing on the contamination of pharmaceutical products by aluminium. Part III: Interaction container-chemicals during heating for sterilisation. J Trace Elem Med Biol. 2003;17:107–115.
- Nicolini M, Zatta P, Corain B. Aluminum in Chemistry, Biology, and Medicine. Verona: EditoraCortina International; 1991.
- Garabed E, Adeera L, Nathan WL. Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):1–201.
- Sharma B, Bader F, Templeman T, Lisi P, Ryan M, Heavner GA. Technical investigations into the cause of the increased incidence of antibody-mediated pure red cell aplasia associated with EPREX®. Eur J Hosp Pharm. 2004;5:86–91.
- Aguilar F, Autrup H, Barlow S, et al. Scientific opinion of the panel on food additives, flavouring, processing aids and food contact materials on a request from European Commission on safety of aluminium from dietary intake. EFSA J. 2008;754:1–34.