Abstract
Nephrotoxicity is a major complication of gentamicin (GEN). We aimed to evaluate the potential protective effect of montelukast (MK) against GEN-induced nephrotoxicity in rats. Thirty-two rats were randomly divided into four groups, each consisting of eight animals as follows: (1) the rats were control; (2) intraperitoneally injected with GEN 14 consecutive days (100 mg/kg/day); (3) treated with GEN plus distilled water via nasogastric gavage for 14 days; and (4) treated with GEN plus MK (10 mg/kg/day) for 14 days. After 15 days, rats were killed and their kidneys were taken and blood analysis was performed. Twenty-four hours urine collections were obtained in standard metabolic cages a day before the rats were killed. Tubular necrosis and interstitial fibrosis scoring were determined histopathologically in a part of kidneys; nitric oxide (NO), malondialdehyde (MDA), and reduced glutathione (GSH) levels were determined in the other part of kidneys. Statistical analyses were made by the chi-square test and analysis of variance. Serum urea and creatinine levels were significantly higher in rats treated with GEN alone, than the rats in control and GEN + MK groups.The GSH levels in renal tissue of only GEN-treated rats were significantly lower than those in control group, and administration of MK to GEN-treated rats significantly increased the level of GSH. The group that was given GEN and MK had significantly lower MDA and NO levels in kidney cortex tissue than those that was given GEN alone. In rats treated with GEN + MK, despite the presence of mild tubular degeneration and tubular necrosis are less severe, and glomeruli maintained a better morphology when compared with GEN group. We can say that MK prevents kidney damage with antioxidant effect, independently of NO.
INTRODUCTION
The kidney is a vital organ, which plays an essential role in health, disease, and overall development and growth. The main function of kidney is to maintain total body fluid volume, its composition, and acid–base balance. A number of environmental variables including certain drugs influence these functions.Citation1–3 Aminoglycoside antibiotics, including gentamicin (GEN), are widely used in the treatment of gram-negative infections. A major complication of these drugs is nephrotoxicity, and it has been estimated that approximately 10–20% of the cases were treated with aminoglycoside therapy.Citation4 Although the majority of aminoglycosides is excreted in the urine after drug administration, approximately 5–10% of the dose accumulates in the renal cortex and remains there long after discontinuation of the drug.Citation5,6 Gentamicin is not metabolized in the body but is essentially eliminated by glomerular filtration and partially reabsorbed by proximal tubular cells.Citation7 Intravenously administered gentamicin is almost entirely eliminated by the kidney, but a small, toxic portion is selectively reabsorbed and accumulates in the proximal renal tubular cells (RTCs).Citation8,9 Lysosomes are the first and obvious site of sequestration and accumulation of gentamicin in proximal tubules. Gentamicin in lysosomes can be released into cytoplasm through drug overloading, direct permeabilization of the lysosomal membrane, or retrograde trafficking through the Golgi apparatus and the endoplasmic reticulum, resulting in cell death.Citation10–12 It was shown that, despite recovery of renal function 30 days after cessation of gentamicin treatment, rats had increased macrophage infiltration, myofibroblast accumulation, and extracellular matrix (ECM) accretion in the kidney.Citation13 The inflammatory and fibrogenic responses to gentamicin were associated with increases in transforming growth factor (TGF-B), endothelin, and angiotensin II levels, implying their involvement in the progression of tubulointerstitial nephritis. It has also been observed that gentamicin administration can induce apoptosis of cultured proximal tubular cells.Citation14 Furthermore, it has been reported that GEN-induced reactive oxygen species (ROS) are essential mediators of its nephrotoxic effects.Citation15,16 This elevation of ROS would stimulate the activation or expression of proinflammatory and proapoptotic mediators, including nuclear factor kappa B (NF-KB), leukocyte adhesion molecules, mitogen-activated protein kinases (MAPKs), and TGF-B1,Citation17–20 contributing to kidney damage induced by gentamicin. Thus, steps to prevent or reverse aminoglycoside-induced kidney injury would have significant clinical value. Several agents and strategies utilizing different mechanisms have been attempted to reduce GEN-induced nephrotoxicity,Citation16,21,22 but a clinically viable approach has not yet been established.
Cellular sources of inflammatory cytokines enhanced in GEN-induced rat models are mainly neutrophils and monocytes. Once neutrophils migrate into the tissue, they release reactive oxygen species, proteases, elastase, myeloperoxidase (MPO), cytokines, and various other mediators.Citation23 Along with the various pro-inflammatory chemokines, cysteinyl leukotrienes (CysLTs), 5-lipoxygenase metabolites of arachidonic acid, are also proven to be potent inflammatory mediators that cause tissue injury. Antileukotriene drugs, that is, leukotriene receptor antagonists and synthesis inhibitors, are a new class of antiinflammatory drugs that have shown efficacy in the treatment of several inflammatory models. A selective reversible CysLT1 receptor antagonist, montelukast (MK), was used in the treatment of asthma and is reported to reduce eosinophilic inflammation in the airways,Citation24,25 while CysLT1 receptor antagonists or biosynthesis inhibitors have been reported to ameliorate ethanol-induced gastric mucosal damageCitation26,27 and experimental colitis.Citation28 Recently, it is shown that the CysLT1 receptor antagonist, MK, ameliorates burn- and sepsis-induced multiple organ damage.Citation29,30
Furthermore, we investigated the effects of MK on GEN-induced renal damage in rat models.
MATERIAL AND METHODS
Experimental Procedure
Gentamicin (GEN) was purchased from Bilim Pharmaceuticals. GEN was dissolved in saline and injected intraperitoneally. MK (MS & D) diluted with saline to a final concentration of 10 mg/kg was administered by oral gavage under light or either anesthesia. Adult male Wistar Albino rats (200–250 g) were housed in clean plastic cages in a temperature and humidity-controlled facility with a constant 12 h light/dark cycle with free access to food and water. The use of animals and the experimental protocol were approved by the Institutional Animal Care and Use Committee and animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals of Research Council.
After a quarantine period of 7 days, 32 rats were randomly divided into four groups, each consisting of eight animals as follows: (1) the rats were control; (2) intraperitoneally injected with GEN 14 consecutive days (100 mg/kg/day); (3) treated with GEN plus distilled water (vehicle) via nasogastric gavage for 14 days; (4) treated with GEN plus MK (10 mg/kg/day) for 14 days. MK was administered immediately after injection of GEN.
Rats were treated for 14 days. After 15 days, rats were killed and their kidneys were taken and blood analysis was performed. Tubular necrosis and interstitial fibrosis scoring were determined histopathologically in a part of kidneys; nitric oxide (NO), malondialdehyde (MDA), and glutathione (GSH) levels were determined in the other part of kidneys. Urea–creatinine, Na+, and K+ levels were investigated at blood analysis.
Biochemical Analysis
Twenty-four hours after the administration of last doses of GEN and MK, on 15th day, rats were anesthetized by intraperitoneal injection of ketamine and sacrified. Twenty-four hours urine collections were obtained in standard metabolic cages a day before the rats were killed. Renal cortical tissues were separated into two parts for biochemical analysis and light microscopic examination. Blood samples were also taken by cardiac puncture to assess the serum levels of urea, creatinine, Na, and K concentrations. The tissues were shock freezing by liquid nitrogen and we just kept in –80° C. In frozen tissues, malondialdehyde (MDA), end product of lipid peroxidation, glutathione (GSH), nonenzymatic antioxidant, total nitrite, and a stable product of nitric oxide (NO) were evaluated biochemically as a means of oxidative stress.
Renal impairment was assessed by serum urea and creatinine levels, as well as by the kidney histology. Serum urea and creatinine levels were determined with an autoanalyzer (Syncron LX20, Ireland) by using commercial Becman Coulter diagnostic kits. Kidney tissue (300 mg) was homogenized in ice-cold tamponade containing 150 mM KCl for determination of MDA. MDA levels were assayed for products of lipid peroxidation. MDA referred to as thiobarbituric acid reactive substance, was measured with thiobarbituric acid at 532 nm in a spectrofluorometer, as described previously.Citation31 GSH was determined by the spectrophotometric method, which was based on the use of Ellman’s reagent.Citation32
Total nitrite (NOx) was quantified by the Griess reactionCitation33 after incubating the supernatant with Escherichia coli nitrate reductase to convert NO3 to NO2. Griess reagent (1 mL 1% sulfanilamide, 0.1% naphthyl-ethylenediamine hydrochloride, and 2.5% phosphoric acid; Sigma Chemical Co., St. Louis, MO, USA) was then added to 1 mL of supernatant. The absorbance was read at 545 nm after a 30-min incubation. The absorbance was compared with the standard graph of NaNO2, obtained from the reduction of NaNO3 (1–100 lmol/L). The accuracy of the assay was checked in two ways; the inter- and intraassay coefficients of variation were 7.52% and 4.61%, respectively. To check conversion of nitrate to nitrite (recovery rate), known amounts of nitrate were added to control plasma samples; these samples were deproteinized and reduced as above.
Histopathological Evaluation
Histopathological evaluation was made in kidney tissues. Paraffin-embedded specimens were cut into 6-μm thickness and stained with hematoxylin–eosin for light microscopic examination using a conventional protocolCitation34 (Olympus, BH-2, Tokyo, Japan). A semi-quantitative evaluation of renal tissues was accomplished by scoring the degree of severity according to previously published criteria.Citation35 All sections of kidney samples were examined for parietal cell hyperplasia, tubular vacuolization, and tubular necrosis. Briefly, minimum of 50 proximal tubules associated with 50 glomeruli were examined for each slide and an average score was obtained. Severity of lesion was graded from 0 to 3 according to the percentage of tubular involvement. Slides were examined and assigned for severity of changes using scores on scale of none (0), mild (1), moderate (2), and severe (3) damage, in which (0) denotes no change; grade (1) changes affecting <25% tubular damage (mild); grade (2) changes affecting 25–50% of tubules (moderate); and grade 3 changes affecting >50% of tubules (severe).
To evaluate kidney fibrosis, specimens obtained from kidney were embedded in paraffin, sectioned at 6-μm sections, and stained with Masson’s trichrome. Specimens were scored after painting with, in brief, (–) no fibrosis, (+) fibrosis in <25% of total kidney tissue (mild), (++) fibrosis in 25% to 50% of total kidney tissue (moderate), and (++ +) fibrosis in >50% of total kidney tissue (serious).Citation36
Statistical Analysis
Results of all groups were shown as mean values ± standard deviation (SD). Statistical analyses of the histopathologic evaluation of the groups were carried out by the chi-square test and biochemical data were analyzed by the one-way analysis of variance (ANOVA). The significance between the two groups was determined by the Dunnett’s multiple comparison test, and p < 0.05 was accepted as statistically significant value.
RESULTS
No deaths or remarkable signs of external toxicity were observed in the groups of rats given GEN either alone or combination with MK. The biochemical and histopathological results were similar for control and MK groups, and we decided to consider them without distinction and report only the control group.
Urinary Volume
The 24-h urine volume in group GEN was significantly higher than in control group (p < 0.01), indicating the presence of GEN-induced polyuria, whereas in group GEN + MK, it was not different from that in control group, pointing out the protective role of MK against acute tubular necrosis ().
Table 1. Effects of GEN alone and its combination with MK on plasma urea, creatinine, Na, K, and 24-h urine volume levels in rats.
Biochemical Variables in Plasma and Tissue
Na+ and K+ concentrations among the four groups were similar. Serum urea and creatinine levels were significantly higher in rats treated with GEN alone than the rats in control and GEN + MK groups (p < 0.01). Administration of MK to the GEN-treated rats caused decrease in serum urea and creatinine levels ().
The GSH levels in renal tissue of only GEN-treated rats were significantly lower than those in control group (p < 0.05), and administration of MK to GEN-treated rats significantly increased the level of GSH (p < 0.05) (, ).
Figure 1. The GSH level in renal tissue of only GEN-treated rats were significantly lower than those in control group, and administration of MK to GEN-treated rats significantly increased the level of GSH. Group given GEN and MK had significantly lower MDA and NO levels in kidney cortex tissue than those given GEN alone.
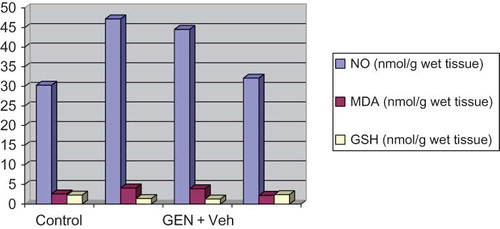
Table 2. Effects of MK on rat kidney NO, MDA, and GSH levels.
The group given GEN and MK had significantly lower MDA levels in kidney cortex tissue than those given GEN alone. There was high level of NO in GEN-treated group; however, NO levels in group treated with GEN + MK were significantly lower than GEN-treated group (, ).
There was no significant difference for biochemical variables between MK and control group ( and ).
Histopathologic Examinations Results
Histopathologic examination of kidney showed that there were no pathologic findings in control group (A). In rats treated with GEN and GEN + vehicle, there were mild and severe tubular necrosis, tubular degeneration, and epithelial vacuolization in the proximal tubules, and parietal cell hyperplasia compared to control group (C and D). In rats treated with GEN + MK, despite the presence of mild tubular degeneration and epithelial vacuolization in the proximal tubules, parietal cell hyperplasia, and tubular necrosis are less severe, and glomeruli maintained a better morphology when compared with GEN group (D). These changes are summarized in .
Figure 2. (A) Normal tubulus and glomerules in kidney cortex H&E 100× (control group). (B) Severe tubular necrosis, tubular degeneration, and epithelial vacuolization in the proximal tubules H&E 100× (GEN-reated group). (C) Moderate tubular necrosis, tubular degeneration, and epithelial vacuolization in the proximal tubules H&E 100× (GEN + vehicle treated group). (D) Mild epithelial vacuolization in the proximal tubules and normal glomerules H&E 100× (GEN + MK treated group).
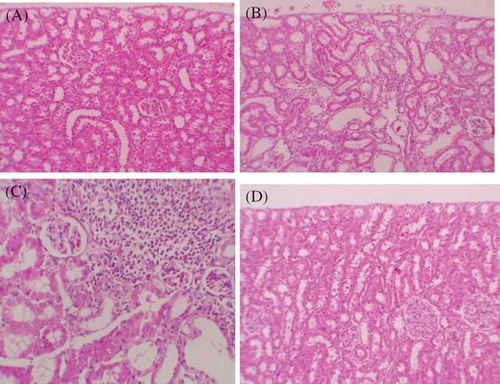
Table 3. Semiquantitative analysis of tubular necrosis, tubular vacuolization, parietal cell hyperplasia in control, GEN, GEN + vehicle, and GEN + MK treated rats.
After staining with Masson trichrome, no statistical difference was found between groups in kidney fibrosis scores. (; A–D).
Figure 3. (A) No fibrosis in control group, staining with Masson’s trichrome 100×. (B) Mild fibrosis in interstitium, staining with Masson’s trichrome 400× (GEN-treated group). (C) Mild fibrosis in interstitium, staining with Masson’s trichrome 200× (GEN + vehicle treated group). (D) No fibrosis in GEN + MK treated group, staining with Masson’s trichrome 100×.
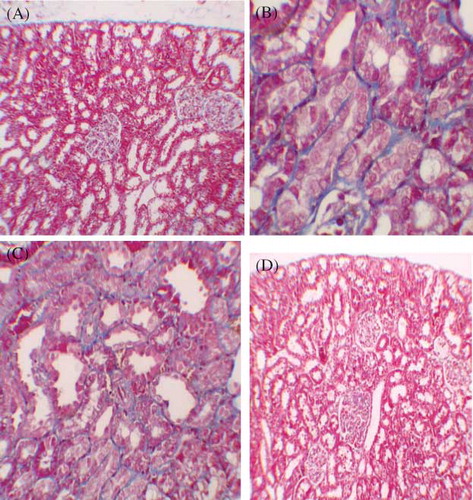
Table 4. Analysis of kidney fibrosis in control, GEN, GEN + vehicle (Ve), and GEN + MK treated rats.
There was no significantly difference for histopathologic examination of kidney between MK and control group ( and ).
DISCUSSION
Kidneys are easily susceptible to damage from drugs because of larger perfusion and increased concentration of excreted compounds that occur in renal tubular cells during absorption and secretion. Aminoglycoside is an antibiotic whose clinical use is limited by its nephrotoxicity. GEN is a widely used aminoglycoside antibiotic and has been shown to cause marked histological damage, in particular to renal proximal convoluted tubulesCitation37,38 resulting in swelling, vacuolization and necrosis of epithelial cells, and accumulation of myelin-like bodies.Citation39
Furthermore, results of many studies have shown that altered concentrations of various biochemical indicators of oxidative stress in kidney tissue are due to GEN.Citation40 Because of the obvious responsibility of ROS in GEN-induced renal damage, several antioxidant agents have been used to prevent GEN nephrotoxicity.Citation41
MK, a selective reversible cys-leukotriene-1 receptor (LTD4 receptor) antagonist, is used in the treatment of asthma and is reported to reduce airway eosinophilic inflammation in this disease.Citation42–44 LTC4 and LTD4 receptor antagonists have also been reported to be beneficial in various inflammatory bowel diseases.Citation45 It was reported by Wallace et al. that an LTC4/LTD4 receptor antagonists ameliorate the ethanol-induced gastric mucosal damage.Citation45,46 In addition, in this study we aimed to examine the possible beneficial effects of an LT receptor blocker, MK, to attenuate the functional, morphological, and histochemical effects displayed in a rat model of GEN nephrotoxicity. The understanding of aminoglycoside nephrotoxicity is clinically important, such as nephrotoxicity is typically associated with nonoliguric acute renal failure, that is, azotemia in the presence of 1–2 L/day urine output. In present study, the 24-h urine volume in the GEN group was significantly higher than in the control group, indicating the presence of GEN-induced polyuria, whereas in GEN + MK group, it was not different from that of the control group, suggesting the protective role of MK against acute tubular necrosis. Also, increased serum urea and creatinine levels in GEN-treated rats reflect the renal damage. In contrast to previous studies, serum K+ levels were similar between group GEN-induced nephrotoxicity and control group. Previous studies show that K+ levels were decreased in rats with GEN-induced nephrotoxicity. It is well documented that GEN nephrotoxicity in experimental animals causes acute renal failure and reduction in serum K+ levels. These results could be attributed to the fact that GEN-induced oxidative stress can promote the formation of a variety of vasoactive mediators that can affect renal function directly by causing renal vasoconstriction or decreasing the glomerular capillary ultrafiltration coefficient and thus reducing glomerular filtration rate (GFR). ROS generated by GEN may also impair the expression of endothelial nitric oxide synthase (eNOS), whereas O2− may scavenge nitric oxide (NO), thereby reducing the amount of the endogenous vasodilator in the vasculature.Citation47,48 Alternatively, the GEN-induced tubular necrosis could lead to a decrease in the number of functioning nephrons, with a subsequent decline in GFR. This effect may trigger multiple adaptive processes in the hyper-functioning remaining nephrons, most notably the alteration in a variety of tubular transport functions including augmented rates of Na+ reabsorption or K+ secretion. The present biochemical finding is confirmed by histopathological examination of GEN-intoxicated kidneys that revealed diffused renal tubular necrosis, associated with periglomerular and perivascular inflammatory cells infiltration, as well as atrophy in the tuft of the glomeruli with thickening in the Bowman’s capsule.Citation47–49 Administration of MK may protect the kidney function from GEN as indicated by decrease in serum urea and creatinine levels.
Antagonizing CysLT1 receptors with MK treatment prevented the elevation of cytokine production and the GEN-induced renal injury, suggesting that leukotrienes may modulate the pro-inflammatory cytokines and the extent of oxidative injury. So here we measured the MDA, GSH, and nitric oxide (NO), as a means of oxidative stress. The pro-inflammatory cytokines activate neutrophils, macrophages or monocytes, platelets, and mastocytes, which release large amounts of the toxic oxidizing reactive oxygen metabolites (ROMs), causing cellular injury via several mechanisms including the peroxidation of membrane lipids, and the oxidative damage of proteins and DNA.Citation50–52 In the present study, malondialdehyde (MDA) levels, which indicate lipid peroxidation of the membranes as a result of oxidative damage,Citation53 were significantly increased after GEN treatment, again demonstrating tissue damage. However, this increase in lipid peroxidation may partly be due to the free radicals generated by neutrophils. MK, that inhibit neutrophil infiltration, also inhibit the lipid peroxidation. In parallel to increases in MPO and MDA levels, GSH levels were decreased in renal tissue. GSH, the main intracellular nonprotein sulfhydryl, plays an important role in the maintenance of protein and lipid integrity, and provides major protection in oxidative injury by participating in the cellular defense systems against oxidative damage.Citation54 Several reports indicate that GSH depletion correlates with tissue injury induced by various stimuli.Citation55,56 On the other hand, the renal NO levels were found to be significantly higher in only GM-treated rats than those in the control group (p < 0.001), and treatment with MK significantly prevented the elevation of NO levels in our study. In accordance with these biochemical changes that verify the antioxidant effect of MK, both the morphologic evaluation and the renal functional tests revealed that MK may also be effective in protecting the kidney against GEN-induced degenerative changes.
Animal models of aminoglycoside nephrotoxicity present residual areas of interstitial fibrosis in the renal cortex and progressive tubular injury.Citation57,58 In this study, the histopathologic examination of kidneys showed severe and extensive damage in GEN-treated rats which had tubular necrosis and edema. This could be due to the formation of highly reactive radicals as a consequence of oxidative stress caused by GEN. On the other hand, the tubules from rats of the GEN + MK group were nearly normal in histological appearance except for a slight desquamation and atrophy of the tubular epithelial cells. Similar changes were also reported by some studies who demonstrated structural changes in renal tissue of gentamicin-treated animals and its reversal by various agents.Citation59–62
In conclusion, the present study clearly demonstrates that CysLTs are one of the principal mediators involved in GEN-induced oxidative tissue damage in both the kidney and other target organs. MK, a CysLT1 receptor antagonist, reduces GEN-induced neutrophil accumulation, oxidative injury, and renal dysfunction. So, MK is a highly free radical scavenger agent, may offer protection against GEN-induced acute renal failure.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Mahmood I, Waters DH. A comparative study of uranyl nitrate and cisplatin induced renal failure in rat. Eur J Drug Metab Pharmacol. 1994;19:327–336.
- Begg EJ, Barclay ML. Aminoglycisides – 50 years on. Br J Clin Pharmacol. 1995;39:597–603.
- Fatima S, Yusufi ANK, Mahmood R. Effect of cisplatin on renal brush border membrane enzymes and phosphate transport. Hum Exp Toxicol. 2004;23:547–554.
- Humes HD, Weinberg JM. Toxic nephropathies. In: Brenner BM, Rector FC, eds. The Kidney. Philadelphia, PA: W.B. Saunders Co.; 1986:1491–1532.
- Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–1012.
- Nagai J, Takano M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokinet. 2004;19:159–170.
- Karadeniz A, Yildirim A, Simsek N, Kalkan Y, Celebi F. Spirulina platensis protects against gentamicin-induced nephrotoxicity in rats. Phytother Res. 2008;22:1506–1510.
- Leehey DJ, Braun BI, Tholl DA, . Can pharmacokinetic dosing decrease nephrotoxicity associated with aminoglycoside therapy. J Am Soc Nephrol. 1993;4:81–90.
- Schmitz C, Hilpert J, Jacobsen C, . Megalin deficiency offers protection from renal aminoglycoside accumulation. J Biol Chem. 2002;277:618–622.
- Servais H, Van Der Smissen P, Thirion G, . Gentamicin-induced apoptosis in LLC-PK1 cells: involvement of lysosomes and mitochondria. Toxicol Appl Pharmacol. 2005;206:321–333.
- Servais H, Jossin Y, Van Bambeke F, Tulkens PM, Mingeot- Leclercq MP. Gentamicin causes apoptosis at low concentrations in renal LLC-PK1 cells subjected to electroporation. Antimicrob Agents Chemother. 2006;50:1213–1221.
- Sandoval RM, Molitoris BA. Gentamicin traffics retrograde through the secretory pathway and is released in the cytosol via the endoplasmic reticulum. Am J Physiol Renal Physiol. 2004;286:F617–F624.
- Geleilete TJ, Melo GC, Costa RS, Volpini RA, Soares TJ, Coimbra TM. Role of myofibroblasts, macrophages, transforming growth factor-beta endothelin, angiotensin-II, and fibronectin in the progression of tubulointerstitial nephritis induced by gentamicin. J Nephrol. 2002;15:633–642.
- El Mouedden M, Laurent G, Mingeot-Leclercq MP, Tulkens PM. Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicol Sci. 2000;56:229–239.
- Walker PD, Barri Y, Shah SV. Oxidant mechanisms in gentamicin nephrotoxicity. Ren Fail. 1999;21:433–442.
- Cuzzocrea S, Mazzon E, Dugo L, . A role for superoxide in gentamicin-mediated nephropathy in rats. Eur. J Pharmacol. 2002;450:67–76.
- Diamond JR, Kees-Folts D, Ding G, Frye JE, Restrepo NC. Macrophages, monocyte chemoattractant peptide-1, and TGF-beta 1 in experimental hydronephrosis. Am J Physiol. 1994;266:F926–F933.
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253.
- Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651.
- Tang WW, Feng L, Mathison JC, Wilson CB. Cytokine expression, upregulation of intercellular adhesion molecule-1, and leukocyte infiltration in experimental tubulointerstitial nephritis. Lab Invest. 1994;70:631–638.
- Ali BH. Agents ameliorating or augmenting experimental gentamicin nephrotoxicity: some recent research. Food Chem Toxicol. 2003;41:1447–1452.
- Nagai J, Takano M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab Pharmacokin. 2004;19:159–170.
- Kelly KJ, Williams WW, Colvin RB, . Intercellular adhesion molecule-1 – deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063.
- Damtew B, Marino JA, Fratianne RB, Spagnuolo PJ. Neutrophil lipoxygenase metabolism and adhesive function following acute thermal injury. J Lab Clin Med. 1993;121:328–336.
- Wallace JL, Beck PL, Morris GP. Is there a role for leukotrienes as mediators of ethanol-induced gastric mucosal damage? Am J Physiol. 1988;254:G117–G123.
- Carsin H, Bargues L, Stephanazzi J, Paris A, Aubert P, Le Bever H. Inflammatory reaction and infection in severe burns. Pathol Biol. 2002;50:93–101.
- Wallace JL, McKnight GW, Keenan CM, Byles NI, MacNaughton WK. Effects of leukotrienes on susceptibility of the rat stomach to damage and investigation of the mechanism of action. Gastroenterology. 1990;98:1178–1186.
- Konturek SJ, Brozozowski T, Drozdowicz D, Beck G. Role of leukotrienes in acute gastric lesions induced by ethanol, taurocholate, aspirin, platelet-activating factor and stress in rats. Dig Dis Sci. 1988;33:806–813.
- Sener G, Kabasakal L, Cetinel S, Contuk G, Gedik N, Yegen B. Leukotriene receptor blocker montelukast protects against burn-induced oxidative injury of the skin and remote organs. Burns. 2005;31:587–596.
- Sener G, Sehirli O,Cetinel S, . Amelioration of sepsis-induced hepatic and ileal injury in rats by the leukotriene receptor blocker montelukast. Prostoglandins Leukot Essent Fatty Acids. 2005;73:453–462.
- Villegas I, Martín AR, Toma W, . Rosiglitazone, an agonist of peroxisome proliferator-activated receptor c, protects against gastricischemia–reperfusion damage in rats: role of oxygen free radicals generation. Eur J Pharmacol. 2004;505:195–203.
- Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence sample preservation and storage. Clin Chem. 1993;39:2522–2528.
- Beutler E. Glutathione in Red Blood Cell Metabolism. A Manual of Biochemical Methods. New York: Grune and Stratton; 1975: 112–114.
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1998;34:497–500.
- Allen CT. Laboratory methods in histochemistry. In: Prophet EB, Mills B, Arrington JB, Sobin LH, eds. American Registry of Pathology. 1st ed. Washington DC: Armed Forces Institute of Pathology; 1992:53.
- Ayyıldız A, Nuhoğlu B, Gülerkaya B, Caydere M, Ustün H, Germiyanoglu C, . Effect of intraurethral mitomycin C on healing and fibrosis in rats with experimentally induced urethral stricture. Int J Urol. 2004;11:1122–1126.
- Humes HD, Connor RPO. Aminoglycoside nephrotoxicity. In: Schrier RW, Gottschalk CW, eds. Diseases of the Kidney. Vol. 2. no. 4. Boston, MA: Little Brown; 1988:1229–1273.
- Abdel-Gayoum AA, Ali BH, Abdel-Razig AA, Bashir AA, Ghywarsha K. Effect of gentamicin-induced nephrotoxicity on some carbohydrate metabolic pathways in the rat renal cortex. Arch Toxicol. 1994;68:643–647.
- Ali BH, Bashir AA. Effect of fish oil treatment on gentamicin nephrotoxicity in rats. Anal Nutr Metab. 1994;38:336–339.
- Humes HD. Aminoglycoside nephrotoxicity. Kidney Int. 1988;33:900–911.
- Simmons CFJr, Bogusky RT, Humes HD. Inhibitory effects of gentamicin on renal mitochondrial oxidative phosphorylation. J Pharmacol Exp Ther. 1980;214:709–715.
- Aharony D. Pharmacology of leukotriene receptor antagonists. Am J Respir Crit Care Med. 1998;157:S214–S219.
- O’Byrne PM. Asthma treatment: antileukotriene drugs. Can Respir J. 1998;5(Suppl. A):64A–70A.
- Wenzel SE. Leukotriene receptor antagonists and related compounds. Can Respir J. 1999;6:189–193.
- Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36.
- Wallace JL, McKnight GW, Keenan CM, Byles NI, MacNaughton WK. Effects of leukotrienes on susceptibility of the rat stomach to damage and investigation of the mechanism of action. Gastroenterology. 1990;98:1178–1186.
- Silan C, Uzun O, Comunoglu NU, Gokcen S, Bedirhan S, Cengiz M. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharm Bull. 2007;30:79–83.
- Cronin RE, Thompson JR. Role of potassium in the pathogenesis of acute renal failure. Miner Electrolyte Metab. 1991;17: 100–105.
- Mohamed Said M. The protective effect of eugenol against gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Fund Clin Pharmacol. 2011;25:708–716.
- Burkhardt S, Reiter RJ, Tan D-X, Hardeland R, Cabrera J, Karbownik M. DNA oxidatively damaged by chromium(III) and H2O2 is protected by the antioxidant melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, resveratrol and uric acid. Int J Biochem Cell Biol. 2001;33:775–783.
- Oberg BP, Mc Menamin E, Lucas FL, . Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic renal disease. Kidney Int. 2004;65: 1009–1016.
- Ece A, Atamer Y, Gurkan F, . Paraoxonae, anti-oxidant response and oxidative stress in children with chronic renal failure. Pediatr Nephrol. 2006;21:239–245.
- Reiter RJ, Acuno-Castroviejo D, Tan DX, Burkhardt S. Free radical-mediated molecular damage, Ann. NY Acad Sci. 2001;939:200–215.
- Ross D. Glutathione, free radicals and chemotherapeutic agents. Pharmac Ther. 1988;37:231–249.
- Paller MS, Patten M. Protective effects of glutathione, glycine, or alanine in an in vitro model of renal anoxia. J Am Soc Nephrol. 1992;2:1338–1344.
- Mandel LJ, Schnellmann RG, William RJ. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest. 1989;85:316–324.
- Houghton DC, English J, Bennett WM. Chronic tubulointerstitial nephritis and renal insufficiency associated with longterm “subtherapeutic” gentamicin. J Lab Clin Med. 1988;112: 694–703.
- Fuhr J, Kaczmarczyk J, Kruttgen CD. A simple colorimetric method of inulin determination in renal clearance studies on metabolically normal subjects and diabetics. Klin Wochenschr. 1955;33:729–730.
- Kumar KV, Shifow AA, Naidu MU, . Carvedilol: a beta blocker with antioxidant property protects against gentamicin-induced nephrotoxicity in rats. Life Sci. 2000;66:2603–2611.
- Nakakuki M, Yamasaki F, Shinkawa T, . Protective effect of human ulinastatin against gentamicin-induced acute renal failure in rats. Can J Phys Pharmacol. 1996;74:104–111.
- Ozbek E, Cekmen M, Ilbey YO, . Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-kB pathways. Renal Fail. 2009;31: 382–392.