Abstract
Background: Encapsulating peritoneal sclerosis (EPS) is characterized by neovascularization, increased inflammation, and interstitial fibrosis of the peritoneum. We investigated the effects of imatinib on the peritoneal membrane in an experimental EPS model. Methods: We separated 24 non-uremic Wistar rats into four groups: the control group which was injected with 2 mL isotonic saline intraperitoneally (IP) daily for 3 weeks, the CG group which was injected with chlorhexidine gluconate (CG) IP daily for 3 weeks, the resting group which was injected with CG IP between weeks 0–3 followed by a peritoneal rest period between weeks 3–6, and the CG + Imatinib mesylate group (CG + IMA) which received CG through weeks 0–3 followed by 50 mg/kg imatinib mesylate through weeks 3–6. At the end of the study, we performed a 1-h-peritoneal equilibration test and examined the peritoneal function and transforming growth factor-β1 (TGF-β1) in dialysate. Morphologic changes were evaluated by microscopy and immunohistochemistry. Results: An increased ultrafiltration, dialysate/plasma-creatinine-ratio, end-to-initial-dialysate-glucose-ratio, decreased active mesothelial cell ratio and inflammation, and a slightly decreased TGF-β1 of dialysate were found in the CG + IMA group compared to CG alone. Furthermore, the CG + IMA group had a lower concentration of active mesothelial cells than did the resting group. Ultrafiltration was improved in CG + IMA group compared to resting group, however, significant decrease in peritoneal thickness and inflammation were not found compared to those in resting group. Furthermore, there was no significant difference in fibrosis or TGF-β1-positivity on immunohistochemistry between the groups. Conclusions: Tyrosine kinase inhibition with imatinib may lead to a decrease in mesothelial cell activity and an increase in ultrafiltration. However, peritoneal fibrosis was unchanged by imatinib in EPS model.
INTRODUCTION
Encapsulating peritoneal sclerosis (EPS) is the most serious complication of peritoneal dialysis treatment. Although uncommon, EPS results in high mortality rates. Histologically, it is characterized by fibrin deposition, proliferation of fibroblasts, neovascularization, and increased inflammation with mononuclear cell infiltration and exaggerated interstitial fibrosis of the peritoneum. Treatment of peritoneal fibrosis with anti-inflammatory/immunosuppressive agents has been evaluated previously, but only a few agents, such as tamoxifen and glucocorticoids, are currently used in clinical practice.Citation1 To increase the utilization of the peritoneal dialysis (PD) modality worldwide, emerging strategies have shifted to the development of novel antifibrotic agents using experimental models. An ideal antifibrotic agent should decrease the production of collagen and reduce the preexisting accumulation of extracellular matrix.
Tyrosine kinases regulate normal cellular processes, including growth, differentiation, and apoptosis. Pathological tyrosine kinase activation may drive fibrogenesis, which is involved in various diseases, including cancer, pulmonary arterial hypertension, and systemic sclerosis.Citation2 Recently, there has been an interest in the efficacy of tyrosine kinase inhibitors in the treatment of fibrotic diseases. Imatinib, the first generation tyrosine kinase inhibitor to be established in the treatment of chronic myeloid leukemia,Citation3 inhibits the tyrosine kinase activity of the c-abl, which interferes with the signaling pathway of transforming growth factor β (TGF-β) and platelet-derived growth factor (PDGF). TGF-β and PDGF are thought to play important roles in fibroblast activation.Citation4,5 Imatinib has also been shown to prevent the development of dermal, pulmonary, renal, and liver fibrosis.Citation6–9 In this study, we investigated the effects of imatinib on the peritoneal membrane in an experimental EPS model.
MATERIALS AND METHODS
We included 24 male 8-week-old non-uremic Wistar albino rats weighing 250–300 g in the present study. All animal procedures were performed under standard conditions in the Marmara University Animal Laboratory, and all experiments conformed to the animal care protocols of the institution. Rats were housed in polycarbonate cages maintained at 24°C with a 12-h light–dark cycle and were fed a standard laboratory diet and given free access to water. The Animal Ethics Committee of Marmara University approved the study design.
Peritoneal fibrosis was induced by intraperitoneal injection of 0.1% chlorhexidine gluconate (CG) in 15% ethanol dissolved in saline, as described previously with some modifications.Citation10 Based on previous reported papers, a 3-week period of treatment with intraperitoneal CG injection was performed.Citation11 To avoid physical damage to the peritoneum caused by repeated injections, CG injections were given in the lower peritoneum, whereas the upper portion was used for histology.
Four groups of rats were used in the experiment: The first group (n = 6), the control group, received 2 mL isotonic saline intraperitoneally (IP), daily for 3 weeks. The second group (n = 6), the CG group, received 2 mL CG solution injected IP, daily for 3 weeks. In the third group (n = 6), the resting group, daily CG injections (weeks 0–3) were followed by peritoneal rest (weeks 3–6), and in the fourth group (n = 6), the CG + Imatinib mesylate (CG + IMA) (Glevec, Novartis, Basel, Switzerland) group , daily CG injections (weeks 0–3) were followed by 50 mg/kg imatinib mesylate diluted in drinking water and delivered via an orogastric tube (weeks 3–6). The dosage of imatinib mesylate was chosen based on the therapeutic effects in other rat models. At the end of the study period, we performed a 1-h peritoneal equilibration test with 25 mL of 3.86% PD solution (Dianeal, Eczacibasi-Baxter, Istanbul, Turkey). After 1 h, rats were anesthetized with ketamine (50 mg/kg body weight), and blood samples were obtained by cardiac puncture immediately. A small midline abdominal incision was made and dialysate samples were collected via a thin silicon catheter for the determination of TGF-β, dialysate-to-plasma ratio of urea (D/P urea), dialysate-to-plasma ratio of creatinine (D/P creatinine), end-to-initial dialysate glucose (D1/D0 glucose), and net ultrafiltration (UF) volumes. Then, the peritoneal cavity was exposed and samples of the parietal peritoneum were taken for further evaluation.
Blood and dialysate fluid levels of glucose, urea, and creatinine were measured with an automatic analyzer. D/P urea, D/P creatinine, and D1/D0 glucose values were calculated. The other portion of the dialysate fluid was stored at –20°C for later measurement of TGF-β concentrations. TGF-β concentrations in dialysate samples were determined independently by antibody enzyme-linked immunosorbent assay (ELISA) using commercial antibody pairs, recombinant standards, and a biotin–streptavidin–peroxidase detection system (Assay Designs, Ann Arbor, MI, USA).
For histologic examination, the tissues were fixed in 10% formalin after collection, then paraffin-processed and embedded. Tissue sections of 4 μm were stained with hematoxylin-eosin and Masson’s trichrome for morphological examination. All histological studies were performed blind by a single pathologist. Neovascularization and inflammation were evaluated based on semi-quantitative scoring. Capillaries and mononuclear cells were counted. Mesothelial cells were classified as normal (flat cells) or reactive (cubic transformation of flat cells), and the proportion of reactive mesothelial cells was used for evaluation. Fibrosis was scored between 0 and 3 regarding early (a few lacy collagens), middle (lacy and mature collagens), and late (mature collagens) phase. Peritoneal thickness was determined as the thickness of the submesothelial zone measured from the inner surface of abdominal muscle to the mesothelium. Thickness was measured with an ocular micrometer using an Olympus microscope (Hamburg/Germany).
For immunohistochemistry of the peritoneum, sections were deparaffinized, rehydrated, and incubated with mouse monoclonal antibodies according to the manufacturer’s directions. Antigen retrieval was used for TGF-β1 (SantaCruz Biotechnology Inc., CA, USA). After incubation, tissue sections were covered with anti-mouse secondary antibody, washed and slides were incubated in peroxidase conjugated with a streptavidin-biotin complex. The areas containing positive cells for TGF-β1 were evaluated under 200× magnification. Results are reported as the percentages of positive cells per mmCitation2 of peritoneal tissue.
In statistical analysis, all continuous variables are expressed as the means ± standard error of the mean and proportions are expressed as numbers (%). Data sets were examined by ANOVA and comparisons among the four groups were performed using Mann–Whitney U-tests for and chi-square tests where appropriate. A value of p < 0.05 was considered to indicate statistical significance.
RESULTS
Peritoneal Function
shows the results of the peritoneal transport tests. CG exposure for 3 weeks resulted in obvious alterations in peritoneal transport characteristics. In the CG group, the net UF volume and D1/D0 glucose levels were significantly decreased as compared to the control group (–0.17 ± 1.6 vs. 7.15 ± 1.2 mL, p < 0.001; 0.45 ± 0.04 vs. 0.77 ± 0.14, p < 0.001; respectively). D/P urea and D/P creatinine ratios were increased with the CG injections, indicating impaired peritoneal transport. Although the net UF volume, which decreased with CG injections, improved with rest, the improvement was not statistically significant (–0.17 ± 1.6 vs. 3.7 ± 4.3, p = 0.109). Peritoneal resting for 3 weeks significantly improved D1/D0 glucose ratios as compared to the CG group (0.72 ± 0.16 vs. 0.45 ± 0.04, p = 0.010). Imatinib administration resulted in better UF volumes as compared to those in the CG and resting groups (6.1 ± 3.8 mL vs. –0.17 ± 1.6 mL, p = 0.037 and 6.1 ± 3.8 mL vs. 3.7 ± 4.3 mL, p = 0.020). However, there were no significant improvements in D/P urea, D/P creatinine, and D1/D0 glucose ratios when compared to those in the peritoneal rest group.
Table 1. Comparison of study groups regarding functional alternations.
Table 2. Comparison of study groups regarding morphologic alterations.
Dialysate TGF-β Level
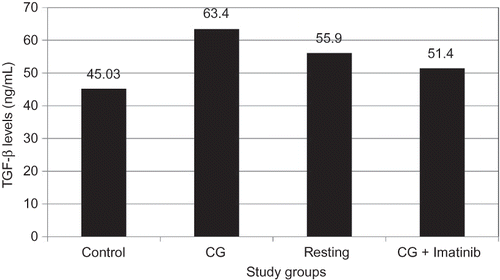
Dialysate TGF-β measurements are shown in . Dialysate TGF-β levels were significantly higher in the CG group as compared to the control group (63.4 ± 10.9 vs. 45 ± 7.7, p = 0.42). In the resting and imatinib treatment groups, TGF-β levels slightly decreased after CG exposure, but the decrease was not statistically significant.
Peritoneal Histopathology
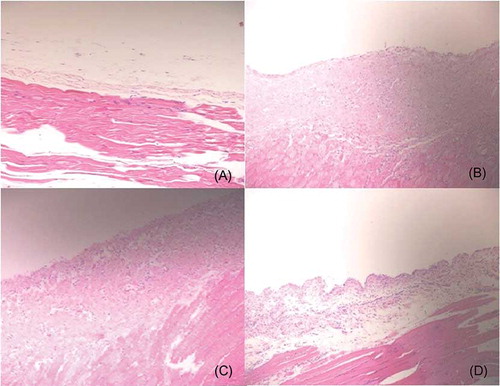
Data on the histopathology of peritoneal samples are summarized in . There were significant morphological alterations in peritoneal membrane characteristics between the study groups. Peritoneal thickness, angiogenesis, and fibrosis score were increased significantly in the CG group as compared to those in the control group. The inflammation score and proportion of reactive mesothelial cells were also elevated in the CG group. Resting had no beneficial effects on structural parameters. However, imatinib administration significantly decreased the proportion of reactive mesothelial cells compared to that in the resting group (16.6% vs. 83.3%, respectively). Peritoneal thickness, number of vessels, inflammation, and fibrosis score did not improve with imatinib therapy ().
Figure 2. Histologic features of the parietal peritoneum (hematoxylin-eosin, magnification ×200). (A) The intact thin mesothelial layer of the peritoneum overlies muscle in the control group. (B) Chlorhexidine significantly increased thickness, cellularity (inflammation), and fibrotic changes. (C) Peritoneal rest had no morphologic benefits; increased vascularity, thickness, and fibrosis are also shown in the resting group. (D) Imatinib treatment group is characterized by reduced cellularity (inflammation).
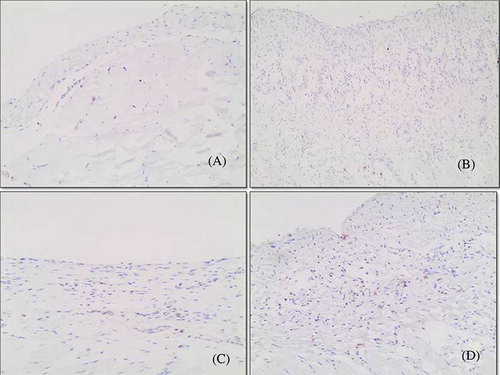
Peritoneal Immunohistochemistry
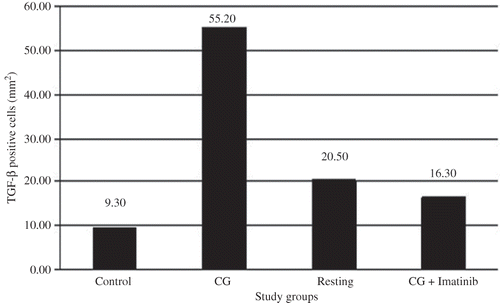
Compared with the control group, rats in the CG group had increased numbers of TGF-β1-positive cells (). The numbers of TGF-β1-positive cells were significantly decreased in the CG + IMA group and the resting group. However, there was no significant difference between the CG + IMA group and the resting group ().
DISCUSSION
In this study, we demonstrate that imatinib treatment exerts beneficial effects in a rat model of EPS. CG-induced EPS is characterized by a marked thickening of the submesothelial zone of the parietal peritoneum, infiltration of inflammatory cells, and increased mesothelial cell reactivity, resulting in significant reduction in ultrafiltration. Our results show that tyrosine kinase inhibition with imatinib improves net UF volume and decreases the proportion of reactive mesothelial cells, which reflects healing of peritoneal injury.
EPS is characterized by activation of peritoneal mesothelial cells, accumulation, and deposition of excess matrix proteins within the interstitium and neovascularization of the peritoneal microstructure.Citation12 Animal models have previously shown an increased expression of TGF-β, alpha- smooth muscle actin (α-SMA), collagen, and vascular endothelial growth factor in the peritoneum. Neovascularization and fibrosis seem to be intimately linked to the initiation of these growth factors, inflammatory cytokines, and epithelial-to-mesenchymal transition (EMT) of mesothelial cells. There is increasing evidence that prevention of EMT or inhibition of growth factors and cytokine release may ameliorate peritoneal fibrosis and angiogenesis.Citation13 Previously, tamoxifen, somatostatin, colchicine, and everolimus have been suggested as therapeutic treatments in EPS animal models.Citation14–17 These focus primarily on treating underlying inflammation and suppression of inflammatory cell activity using anti-inflammatory therapies in conjunction with immune-modulating drugs. Unfortunately, only tamoxifen has been proven to be effective in the treatment of EPS. Imatinib mesylate is a known potent inhibitor of TGF-β and PDGF, which has been used as an antifibrotic agent in bleomycin-induced pulmonary and dermal fibrosis rat models.Citation4,5 However, in our study, imatinib failed to inhibit progression of peritoneal fibrosis.
Imatinib may have failed to inhibit progression of fibrosis in this EPS model for several reasons. Firstly, previous studies show that the antifibrotic effects of this agent depend largely on the period of administration. Regression of established fibrosis is probably mediated by inhibition of collagen synthesis and/or increase in matrix degradation. Imatinib administration during the post-injury reparation phase does not protect against fibrosis,Citation18 probably because Imatinib fails to inhibit TGF–β/SMAD signaling and seems to promote differentiation of myofibroblasts, which continue to express α-SMA. In contrast, administration of imatinib during the injury phase can suppress TGF-β-induced myofibroblast differentiation by SMAD-independent pathways.Citation4
In our study, we also determined the concentrations of TGF-β in dialysate and tissue TGF-β1 expression. Whereas most animal studies have demonstrated a significant reduction in dialysate and tissue TGF-β level after EPS treatment, imatinib therapy had no such effect. These results seem to contradict the hypothesis that imatinib has anti-inflammatory effects. However, insufficient decrease in this cytokine could be due to increased peritoneal mesothelial cell mass, which might be responsible for increased secretion of TGF-β, after the use of imatinib. Indeed, peritoneal mesothelial cells constitutively produce low levels of TGF-β, as well as other factors such as bone morphogenic proteins.Citation19 Tejde et al. Citation20 also showed that there was no decrease in TGF-β levels in dialysate despite improvement in CA 125 levels, which has been identified to correlate with mesothelial cell mass and turnover after the use of bicarbonate containing dialysis fluid in PD patients.
We know that several important properties of mesothelial cells enable homeostasis of the peritoneum by several actions, including regulation of solute and water transport, regulation of peritoneal fibrinolysis, and production and remodeling of the extracellular matrix. Mesothelial cells can facilitate the transport and movement of fluid and solutes across the peritoneal membrane and are the first line of defense during bacterial or chemical inflammation.Citation21 In human peritoneal mesothelial cells, expression of aquaporin-1 and aquaporin-3, which play major roles in transcellular water movement, was demonstrated previously. In line with this data, we thought that improved mesothelial cell activity provides a better UF capacity via aquaporins.Citation22,23 Although the fibrosis score and neoangiogenesis were not improved with imatinib administration, better preservation of the UF volume could be explained by decreased inflammation and decreased mesothelial cell activity.
In this study, we selected a dose of imatinib that is comparable with that used in previous animal models of fibrosis.Citation24 Chaudhary et al. used a bleomycin injury model in male Wistar rats and compared the effects of three doses of imatinib (10, 30, and 50 mg/kg/day); a dose of 50 mg/kg/day was the most efficacious in their study. The recommended dose of imatinib mesylate in chronic myeloid leukemia and gastrointestinal stromal tumors is 400–600 mg/day or 5–8 mg/kg/day, for an average 70 kg human. The doses of imatinib in rats are much higher than those used in humans, which reflects differences in the in vitro activity between species.
The major limitation of the present study was the lack of determination of tissue tyrosine kinase levels. However, this study provides information regarding the beneficial effects of imatinib on the peritoneal membrane, such as improved UF capacity.
Recurrent peritonitis, bioincompatible dialysis solutions, or conventional dialysis solutions with a high glucose concentration and long-term PD treatment can lead to peritoneal fibrosis. EPS is characterized by exaggerated fibrosis of the peritoneum. Although the risk factors for EPS development are well known, EPS occurs in few PD patients and the underlying etiology is unclear. Although a long-term PD duration is one of the risk factors, the onset duration of EPS cannot be predicted. Therefore, the co-treatment model could be favorable for EPS treatment in experimental nephrology; however, further studies are needed before it is implemented in clinical practice.
In conclusion, we demonstrated that tyrosine kinase inhibition with imatinib may improve UF capacity and decrease mesothelial cell activity. However, peritoneal fibrosis was unchanged upon imatinib administration in this experimental EPS model.
Declararion of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Balasubramaniam G, Brown EA, Davenport A, . The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant. 2009;24(10): 3209–3215.
- Beyer C, Distler JH, Distler O. Are tyrosine kinase inhibitors promising for the treatment of systemic sclerosis and other fibrotic diseases? Swiss Med Wkly. 2010;140:w13050, doi:10.4414/smw.2010.13050.
- Druker BJ, Talpaz M, Resta DJ, . Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14): 1031–1037.
- Daniels CE, Wilkes MC, Edens M, . Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004; 114(9):1308–1316.
- Distler JH, Distler O. Tyrosine kinase inhibitors for the treatment of fibrotic diseases such as systemic sclerosis: towards molecular targeted therapies. Ann Rheum Dis. 2010;69(Suppl. 1):i48–i51.
- Akhmetshina A, Venalis P, Dees C, . Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis. Arthritis Rheum. 2009;60(1):219–224.
- Abdollahi A, Li M, Ping G, . Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201(6):925–935.
- Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19(1):1–11.
- Yoshiji H, Noguchi R, Kuriyama S, . Imatinib mesylate (STI-571) attenuates liver fibrosis development in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288(5):G907–G913.
- Hoff CM. Experimental animal models of encapsulating peritoneal sclerosis. Perit Dial Int. 2005;25(Suppl. 4):S57–S66.
- Io H, Hamada C, Ro Y, . Morphologic changes of peritoneum and expression of VEGF in encapsulated peritoneal sclerosis rat models. Kidney Int. 2004;65:1927–1936.
- Di Paolo N, Nicolai GA, Garosi G. The peritoneum: from histological studies to mesothelial transplant through animal experimentation. Perit Dial Int. 2008;28(Suppl. 5):S5–S9.
- Yu MA, Shin KS, Kim JH, . HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J Am Soc Nephrol. 2009;20(3):567–581.
- Huang JW, Yen CJ, Wu HY, . Tamoxifen downregulates connective tissue growth factor to ameliorate peritoneal fibrosis. Blood Purif. 2011;31(4):252–258.
- Ertilav M, Hur E, Bozkurt D, . Octreotide lessens peritoneal injury in experimental encapsulated peritoneal sclerosis model. Nephrology (Carlton). 2011;16(6):552–557.
- Bozkurt D, Bicak S, Sipahi S, . The effects of colchicine on the progression and regression of encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28(Suppl. 5):S53–S57.
- Duman S, Bozkurt D, Sipahi S, . Effects of everolimus as an antiproliferative agent on regression of encapsulating peritoneal sclerosis in a rat model. Adv Perit Dial. 2008;24: 104–110.
- Vittal R, Zhang H, Han MK, Moore BB, Horowitz JC, Thannickal VJ. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/mesenchymal phenotypes: implications for treatment of fibrotic diseases. J Pharmacol Exp Ther. 2007;321(1):35–44.
- Strippoli R, Benedicto I, Perez Lozano/m L, . Inhibition of transforming growth factor-activated kinase 1 (TAK1) blocks and reverses epithelial to mesenchymal transition of mesothelial cells. PLoS One 2012;7(2):e31492.
- Tejde M, Hadimeri H, Siegert CE, . Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int. 2009;29(6):647–655.
- Yung S, Chan TM. Mesothelial cells. Perit Dial Int. 2007;27(Suppl. 2):S110– S115.
- Lai KN, Lam MF, Leung JC. Peritoneal function: the role of aquaporins. Perit Dial Int. 2003;23(Suppl. 2):S20–S25.
- Liakopoulos V, Zarogiannis S, Hatzoglou C, . Inhibition by mercuric chloride of aquaporin-1 in the parietal sheep peritoneum: an electrophysiologic study. Adv Perit Dial. 2006;22: 7–10.
- Chaudhary NI, Roth GJ, Hilberg F, . Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29(5):976–985.