Abstract
Background: The aim of this study is to investigate the clinical characteristics and our experience of treating patients with IgA nephropathy (IgAN) and IgA nephropathy with hepatitis B surface antigen (HBs-IgAN). Methods: From 1996 to 2011, biopsy-proven IgAN was diagnosed in 477 patients and 22 (4.6%) had hepatitis B surface antigen (HBsAg). Of these, we included 360 patients who had more than 6-month follow-up period, and compared clinical characteristics and renal function decline between the patients with IgAN and HBs-IgAN. Results: Of 360 patients, 22 were classified as HBs-IgAN. There were no differences in the clinical characteristics and renal function decline between idiopathic IgAN and HBs-IgAN (–0.01 vs. –0.17 mL/min per 1.73 m2/month, p = 0.319). Of 22 patients with HBs-IgAN, nine had hepatitis B virus (HBV) replication marker (RM), of which six were treated with anti-viral agents. However, there were no differences in renal function decline and urinary protein excretion between patients who did or did not receive anti-viral therapy. Five patients with HBs-IgAN received corticosteroid therapy. Of these, three without HBV RM and one with HBV RM who received entecavir did not exhibit active viral replication, whereas the other patients with HBV RM experienced viral replication after lamivudine was discontinued. Conclusion: There were no differences in the clinical characteristics and prognosis between the patients with IgAN and HBs-IgAN. Further, there were no differences in renal function decline and urinary protein excretion between patients with and without anti-viral therapy. Anti-viral therapy may be considered for treating patients with HBs-IgAN receiving immunosuppressants according to HBV RM.
INTRODUCTION
IgA nephropathy (IgAN) is one of the most common glomerular diseases worldwide. IgAN is an immune complex glomerulonephritis that involves intense IgA1 deposition within the mesangium of the glomerulus.1 Some authors have reported a higher prevalence of the hepatitis B surface antigen (HBsAg) in IgAN in endemic area, suggesting that HBsAg plays a significant role in the pathogenesis of IgAN.Citation2 On the other hand, some researchers have reported that there is no increased prevalence of HBs antigenemia in patients with IgAN and that the HBsAg is not associated with the pathogenesis of IgAN.3
The association of membranous nephropathy (MN) with HBs antigenemia has been well established in endemic regions,Citation4 although the etiological role of hepatitis B virus (HBV) antigenemia and HBV antigen deposition in IgAN remains speculative.Citation5,6 Researchers who suggest a strong association between IgAN and HBs antigenemia indicate that mesangial and subendothelial trapping of circulating immune complexes and subendothelial deposits results in the onset of IgA nephropathy with hepatitis B surface antigen (HBs-IgAN).Citation7–9
In case of HBV-associated membranous nephropathy (HBV-MN), unlike the prognosis in affected children, that in adults is not favorable.Citation10 Immunosuppressants have been used for the basic treatment of MN and can cause deleterious hepatic flare or even fatal decompensation by enhancing viral replication when the drugs are discontinued,Citation11 whereas lamivudine treatment has been reported to be effective for treating HBV-MN.Citation12 However, only little data are available about the clinical characteristics, prognosis, and treatment of HBs-IgAN.
Therefore, we investigated the incidence of HBs-IgAN and compared the clinical characteristics, prognosis, and treatment experience of patients with HBs-IgAN and those with idiopathic IgAN.
METHODS
Patient Selection
From 1995 to 2011, biopsy-proven IgAN was diagnosed in 477 patients. Of these, 22 (4.6%) were found to have serum HBsAg at the time of renal biopsy. One patient whose follow-up period was less than 6 months was excluded from this study. Therefore, a total of 360 patients were enrolled in this study and divided into two groups: the idiopathic IgAN group had no HBsAg (N = 338), and the second group of patients (HBs-IgAN) had IgAN and HBsAg (N = 22). This study was approved by the Institutional Review Board of St. Mary’s Hospital, Seoul.
Clinical and Laboratory Information
The patients’ baseline demographic, clinical, and laboratory data were reviewed at the time of renal biopsy. The renal survival time was defined as the duration from the first assessment to end-stage renal disease (ESRD). ESRD was defined as an eGFR of less than 15 mL/min per 1.73 m2 or condition requiring long-term renal replacement therapy. The eGFR was estimated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation.Citation13 The eGFR value obtained at the first assessment was subtracted from that at the end of the follow-up period and divided by the number of months of follow-up. The resulting value is expressed as the eGFR decline per month and represents the rate of loss of renal function.
Serum HBsAg, anti-HBs, Hepatitis B e antigen (HBeAg), and anti-HBe antibodies were evaluated by radioimmunoassay (Abbott Laboratories, Chicago, IL, USA). Serum HBV DNA levels were measured using a Quantiplex branched DNA assay (Bayer Diagnostics, Berkeley, CA, USA; sensitivity >7 × 105 copies/mL), VERSANT 3.0 branched DNA assay (Bayer HealthCare LLC, Tarrytown, NY, USA; sensitivity >2 × 103 copies/mL), or real-time polymerase chain reaction (PCR; Abbott Real-Time HBV Quantification kit; Abbott Molecular, Inc., Abbott Park, IL; sensitivity >28 copies/mL).
Renal Biopsy
Renal tissue samples were routinely processed and stained with hematoxylin-eosin (H&E), periodic acid-Schiff (PAS), Masson’s trichrome, Jones’s silver, and Congo red stains. For direct immunofluorescence, frozen sections of fresh tissue samples were stained with antisera against human C3, C4, C1q, IgA, IgM, IgG, and fibrinogen. Demonstration of HBV-specific antigen was performed by indirect immunofluorescence using a rabbit polyclonal antibody against HBsAg (Santa cruz,CA, USA) and HBcAg (Dakopatts, Copenhagen, Denmark) according to methods described previously.Citation14 A pathologist diagnosed IgAN using three microscopic analyses of the specimens and categorized the observed lesion into one of five classes according to the World Health Organization (WHO) morphological criteria.Citation15
Statistical Analysis
All the data are presented as mean ± standard deviation unless otherwise specified. Student’s t test and analysis of variance (ANOVA) followed by Scheffe test were carried out where appropriate. Categorical groups were compared by the chi-square test or Fisher exact test, as appropriate. The cumulative percentage of patients who went into renal failure requiring replacement therapy was calculated with the time table method. A p value of <0.05 was considered statistically significant. Statistical analysis was carried out using SPSS version 18.0.
RESULTS
Baseline Characteristics
The baseline characteristics of the 360 study subjects are presented in . Of the study participants, 206 (57%) were male. The mean estimated glomerular filtration rate (eGFR) was 79 mL/min/1.73 m2. The mean follow-up duration was 72 months (range, 6–192), and 24 patients (6.6%) progressed to ESRD requiring renal replacement therapy. Of the 360 patients enrolled in this study, 22 had HBsAg at the time of kidney biopsy and were classified in the HBs-IgAN group. Except for the plasma alanine aminotransferase (ALT) concentrations, there were no differences in the baseline characteristics of idiopathic IgAN (N = 338) and HBs-IgAN (N = 22) groups (). According to the WHO classification, there were no histological differences between idiopathic IgAN and HBs-IgAN (). Out of 22 patients with HBs-IgAN, immunohistochemical staining for HBsAg and HBcAg was performed in the renal tissue of the 10 patients including all nine patients with HBV replication marker. Of these, one case was stained by anti-HBsAg (10%), which was demonstrated in the cytoplasm of tubular cells. HBcAg was not detected in all the 10 biopsy specimens ().
Figure 1. Comparison of histology based on WHO classification between idiopathic IgAN and HBV-IgAN. There was no difference in histology between two the groups.
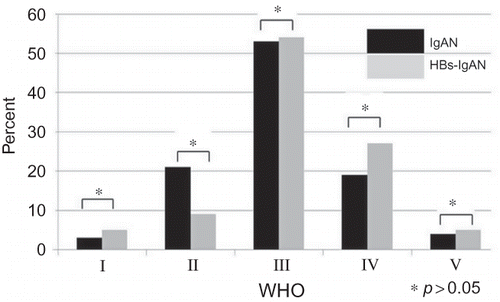
Figure 2. Immunohistochemical staining for HBsAg. Nine tissues were not stained by HBsAg as like A, one case (B) was stained by anti-HBsAg in the cytoplasm of tubular cells.
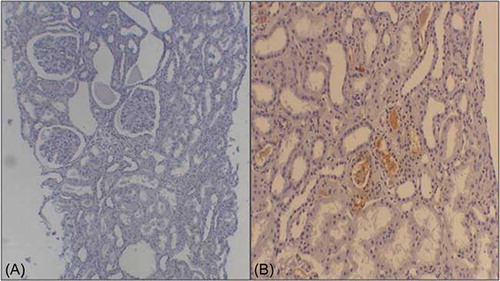
Table 1. The clinical and laboratory findings of the 360 patients with HBs-IgAN.
Table 2. Comparison of baseline characteristics between idiopathic IgAN and HBs-IgAN.
Clinical Course
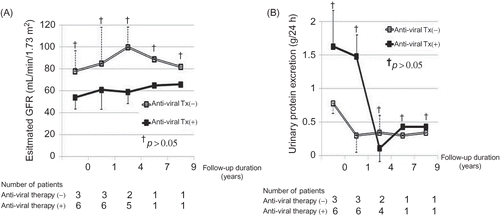
As shown in , the slope of renal function decline was comparable between the idiopathic IgAN and HBs-IgAN patient groups. Comparison of the renal survival between the idiopathic IgAN and HBs-IgAN groups revealed no difference (). Although the enrolled patients had been divided into three groups, namely idiopathic IgAN, HBs-IgAN with HBV replication marker (RM) such as HBV DNA or HBeAg, and HBs-IgAN without HBV RM, there was no difference in the renal survival among the three groups (). In this study, nine patients had HBV RM, and the characteristics of these patients are described in . Of these, six received anti-viral agents. All the patients who received anti-viral therapy exhibited virological response, i.e., seroconversion to anti-HBeAg. However, there were no differences in the slope of renal function decline and urinary protein excretion between patients with (N = 6) and without (N = 3) anti-viral therapy ().
Figure 3. (A) Comparison of renal survival between idiopathic IgAN and HBs-IgAN. There was no difference of renal survival between two groups. (B) Comparison of renal survival between three groups: a group with idiopathic IgAN, a group who had HBs-IgAN without HBVRM and a group had HBs-IgAN with HBV RM. There was no difference of renal survival between three groups.
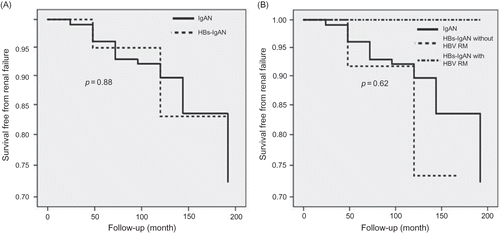
Table 3. The clinical and laboratory findings of 9 patients with HBV RM.
Table 4. Characteristics of patients with steroid therapy.
Out of 22 patients with HBs-IgAN, five received corticosteroid. Of these five patients, two had HBV RMs (). During steroid therapy, no hepatic flare occurred in the three patients without HBV RM. Among the remaining two patients with HBV RMs, the patient who received anti-viral agents during the steroid therapy showed no active viral replication, whereas the other patient maintained stable liver function during lamivudine treatment despite steroid treatment, but experienced active viral replication after lamivudine was discontinued
.DISCUSSION
The etiological role of HBV antigenemia and HBV antigen deposition in IgAN remains controversial. Lai et al. reported that 21 (17.2%) of 122 patients with IgAN had HBs antigenemia and suggested that HBV may play a significant role in the development of IgAN in endemic region.Citation2 On the other hand, Iida et al. reported that only 4 (3.1%) of 130 patients with IgAN had HBs antigenemia and they concluded that HBsAg play an insignificant role, if any, in the pathogenesis of IgAN.3 In our study, 22 (4.6%) of the 477 study subjects with IgAN had HBs antigenemia; this was slightly lower than the prevalence of HBsAg carriers in the general population, which was reported to be 5.7% in the 1990s.Citation16 The detection rate of HBV-specific antigens was 10% in this study (), which was lower than that (59%) of previous reports.Citation2 Thus, it does not appear likely that HBV plays an important role in the pathogenesis of IgAN.
There are little data concerning the clinical characteristics, prognosis, and treatment of HBs-IgAN. Comparing the clinical characteristics between idiopathic IgAN and HBs-IgAN, there were no differences in clinical characteristics except plasma ALT concentration. Lai et al. reported that 1 (4.7%) of the 21 patients with HBs-IgAN developed ESRD requiring hemodialysis during the 40-month follow-up period. In our study, 2 (9%) of the 22 patients with HBs-IgAN required maintenance dialysis. This value is slightly higher than that (6.5%) of idiopathic IgAN, however which was statistically insignificant. The slope of renal function decline (–0.01 vs. –0.17 mL/min per 1.73 m2/month, p = 0.319) was also comparable between idiopathic IgAN and HBs-IgAN. Thus, it seems to be that the prognosis of HBs-IgAN was similar to that of idiopathic IgAN, unlike HBV-MN.
There have been no reports regarding its effectiveness in the treatment of HBs-IgAN. In case of HBV-MN, remission of proteinuria was associated with seroconversion to anti-HBeAg.Citation17,18 In this study, although seroconversion to anti-HBeAg occurred in all patients receiving anti-viral therapy, the slope of renal function decline and urinary protein excretion did not differ between patients with and without anti-viral therapy. Thus, anti-viral therapy did not appear to benefit patients with HBs-IgAN in our study. These results question the role of HBV in the pathogenesis of IgAN.
Steroid and cytotoxic drugs are possibly useful in progressive IgAN.Citation19,20 Five of our study subjects with HBs-IgAN were treated using steroid therapy. One patient with HBV RM experienced reactivation of hepatitis B during steroid treatment. Such active viral replication occurred after lamivudine treatment was discontinued during steroid therapy. On the other hand, the other patient with HBV RM who received entecavir did not experienced active viral replication. Therefore, for treating HBs-IgAN with HBV RM, it is necessary to include anti-viral therapy to prevent viral replication. In our experience, prophylactic anti-viral therapy for patients with HBV RM should be maintained throughout corticosteroid therapy.
In conclusion, the incidence of HBs-IgAN was similar to the prevalence of general population in our study. There were no differences in the clinical characteristics and prognosis between idiopathic IgAN and HBs-IgAN. Further, there were no differences in the renal function decline and urinary protein excretion between patients who did and did not receive anti-viral therapy. Anti-viral therapy may be considered for treating the patient with HBs-IgAN receiving immunosuppressive drugs according to HBV RM.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Rifai A, Millard K. Glomerular deposition of immune complexes prepared with monomeric or polymeric IgA. Clin Exp Immunol. 1985;60:363–368.
- Lai KN, Lai FM, Tam JS, Vallence-Owen J. Strong association between IgA nephropathy and hepatitis B surface antigenemia in endemic areas. Clin Nephrol. 1988;29:229–234.
- Iida H, Izumino K, Asaka M, Fujita M, Takata M, Ig SS. A nephropathy and hepatitis B virus. Nephron. 1990;54:18–20.
- Johnson RJ, Couser WG. Hepatitis B infection and renal disease: clinical, immunopathogenetic and therapeutic considerations. Kidney Int. 1990;37:663–676.
- Wang NS, Wu ZL, Zhang YE, Guo MY, Liao LT. Existence and significance of hepatitis B virus DNA in kidneys of IgA nephropathy. World J Gastroenterol. 2005;11:712–716.
- Wang NS, Wu ZL, Zhang YE, Liao LT. Role of hepatitis B virus infection in pathogenesis of IgA nephropathy. World J Gastroenterol. 2003;9:2004–2008.
- Endo Y, Kanabayashi H. Etiology of IgA nephropathy syndrome. Pathol Int. 1994;44:1–3.
- Barratt J, Feehally J, Smith AC. Pathogenesis of IgA nephropathy. Semin Nephrol. 2004;24:197–217.
- Galla JH. IgA nephropathy. Kidney Int. 1995;47:377–387.
- Lai KN, Li PK, Lui SF, . Membranous nephropathy related to hepatitis B virus-associated nephropathy in adults. N Engl J Med. 1991;324:1457–1463.
- Lai KN, Tam JS, Lin HJ, Lai FM. Therapeutic dilemma of the usage of corticosteroid in patients with membranous nephropathy and persistent hepatitis B virus surface antigenaemia. Nephron. 1990;54:12–17.
- Tang S, Lai FM, Lui YH, . Lamivudine in hepatitis B-associated membranous nephropathy. Kidney Int. 2005;68:1750–1758.
- Levey AS, Greene T, Kusek JW. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828.
- Lai KN, Lai FM, Tam JS. Comparison of polyclonal and monoclonal antibodies in determination of glomerular deposits of hepatitis B virus antigens in hepatitis B virus-associated glomerulonephritides. Am J Clin Pathol. 1989;92:159–165.
- Ig SR. A mesangial nephropathy: Berger’s disease. Am J Nephrol. 1985;5:73–83.
- Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver disease in Korea: hepatitis B. Korean J Hepatol. 2009;15:S13–S24.
- Ito H, Harrori S, Matsuda I, . Hepatitis B e antigen mediated membranous glomerulonephritis. Lab Invest. 1981;44:214–220.
- Lai AS, Lai KN. Viral nephropathy. Nat Clin Pract Nephrol. 2006;2:254–262.
- Pozzi C, Andrulli S, Del Vecchio L, . Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157–163.
- Ballardie FW, Roberts ISD. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–148.