Abstract
Objective: There are some data regarding the role of cystatin C, a cysteine proteinase inhibitor, in determining the glomerular filtration rate (GFR) more accurately. We aimed to evaluate the correlation of serum cystatin C levels with the serum creatinine levels and GFR calculated by Cockcroft–Gault and modification of diet in renal disease (MDRD) formulations in the patients who received cisplatin-based chemotherapy. We also intended to demonstrate its potential use in the early prediction of the renal function changes in these patients. Materials and methods: In the study, 34 patients receiving cisplatin-based chemotherapy with various malignancies were included. The levels of cisplatin were determined prior to the chemotherapy and at the end of cisplatin infusion during the therapy. GFR was calculated by Cockcroft–Gault and MDRD formulations prior to the therapy and at the end of the third course. Results: A statistically significant linear correlation was found between the serum levels of cystatin C and creatinine prior to the chemotherapy (r = 0.42, p = 0.013). However, there was no correlation among the level of cystatin C subsequent to the cisplatin infusion and serum creatinine level following the third course and MDRD and creatinine clearance–Cockcroft–Gault formulations. Conclusion: Even though the serum cystatin C levels were correlated with the serum creatinine levels in our study, it was concluded that it was not an appropriate parameter to predict the potential impairments in the renal function during the chemotherapy.
INTRODUCTION
The alterations in the renal functions should be closely monitored to predict the renal damage early during the use of chemotherapeutics.Citation1 Cisplatin, which is frequently used in the cancer treatment combinations, leads to renal damage due to renal tubular necrosis. Since this effect is cumulative and dose-dependent, it is used in limited doses.Citation2
The glomerular filtration rate (GFR), which is used in monitoring the renal functions, is calculated with the clearance of an exogenous substance such as inulin, iothalamate, diethylenetriamine pentaacetic acid (Cr-EDTA), and iohexol.Citation3 However, since GFR is not a simple and cost-effective calculation method, it cannot be performed at every center. Instead, the serum creatinine level and creatinine clearance are preferred in assessing the renal function.Citation4 Nonetheless, these methods have some limitations, such as the need of secretion of creatinine from the glomerulus and the patients' incompatibility in collecting 24-h urine sample.Citation5 The Cockcroft–Gault and modification of diet in renal disease (MDRD) formulations, taking into account the age, gender, race, and muscle mass, were developed to make the serum creatinine–based GFR measurements more sensitive and simple.Citation6
In recent years, data have shown that cystatin C, a cysteine protein kinase inhibitor, determines GFR more accurately.Citation7–9 Cystatin C is constituted of 120 amino acids and belongs to the cystatin superfamily. It is found in a constant concentration in all cells with nucleus and is excreted to the blood stream.Citation9–11 It is filtrated from the glomeruli without being significantly secreted from the tubuli and is reabsorbed from the proximal tubuli and catabolized.Citation11 Previous studies have reported that the cystatin C level in the individuals with chronic renal disease was affected by the use of glucocorticoids and thyroid dysfunction; on the other hand, inflammatory processes, age, race, gender, and the muscle mass had no effect on the cystatin C level in these individuals.Citation12–15 Some studies have demonstrated that cystatin C was a better indicator in determining the renal function compared to creatinine in the patients receiving cisplatin-based chemotherapy.Citation8,Citation16–18 Furthermore, it was reported that cystatin C was found in higher concentrations in the tissues and body fluids of breast and prostate cancer patients and this was correlated with the aggressive form of the cancer.Citation19,Citation20
The aim of our study was to evaluate the correlation of the serum cystatin C levels with the serum creatinine levels and GFR calculated by Cockcroft–Gault and MDRD formulations in the patients who received cisplatin-based chemotherapy. We also intended to demonstrate its potential use in the early prediction of the renal function changes in these patients.
MATERIALS AND METHODS
Patients and Treatments
A total of 34 patients with various malignancies, who underwent cisplatin-based chemotherapy for adjuvant and palliative treatment, were included in this study. None of the patients received any chemotherapy previously. All the patients were screened for renal failure and thyroid diseases, and the patients with these diseases were excluded from the study. Based on the malignancy types, five different chemotherapeutic regimens were administered. The dose and administration route of modified DCF (mDCF), CF, CFF cisplatin-taxotere, and gemcitabine-cisplatin protocols are shown in . In order to determine the creatinine levels, blood samples were obtained before the treatment, at the end of cisplatin infusion, and after the completion of the third cycle. The cystatin C levels were obtained before the chemotherapy and at the end of cisplatin infusion. Personal information such as weight, age, and gender was gathered at the beginning of the first course and following the third course in order to be used in the Cockcroft–Gault and MDRD formulary.
Table 1. Chemotherapeutic regimens, doses, and protocols.
Laboratory Methods and Formulations
The serum creatinine levels were determined with the DXC-800 analyzer (Beckman-Coulter Instruments, Brea, CA USA) using Jaffe method. The reference range was 0.6–1.3 mg/dL. The serum cystatin C levels were measured with Roche automated chemistry analyzer by immunoturbidimetric method, and the level between 0.47 and 1.09 mg/L was considered as normal.
The creatinine clearance was calculated by the Cockcroft–Gault formulation with the following equationCitation21:
R coefficient is 1 for males and 0.85 for females.
The following equation was used to calculate GFR using MDRDCitation22:
R coefficient is 1 for males and 0.742 for females.
Statistical Analysis
The demographic data and findings were presented as standard deviations (SD) and 95% confidence intervals (CI), and means and medians. The variables were compared using Mann–Whitney test. The statistical differences among the measurements were analyzed by Student's t-test. Correlations were evaluated with parametric Pearson and nonparametric Spearman correlation tests. A p-value less than 0.05 was considered statistically significant. The data were analyzed using Statistical Packages for Social Sciences (SPSS version 13.0).
RESULTS
A total of 34 patients, 20 (58.8%) males and 14 (41.2%) females, were included in the study. The median age was 54 years (range 14–70). Of these patients, 20 (58.8%) had gastric cancer and this was the most frequent malignancy in this patient group. The other malignancies were head and neck cancers (n = 8, 23.5%), lung cancer (n = 3, 8.8%), esophagus cancer (n = 2, 5.9%), and cholangiocellular cancer (n = 1, 2.9%). Fifteen (41.1%) patients received adjuvant therapy, and nineteen patients had metastasis at the time of diagnosis. Based on their diagnosis and the stages, 21 patients (61.8%) received DCF, 7 patients (20.6%) received CFF, 3 patients (8.8%) received CF, 2 patients (5.9%) received gemcitabine–cisplatin, and 1 patient received cisplatin–taxotere treatment protocol ().
Table 2. Patient characteristics (n = 34).
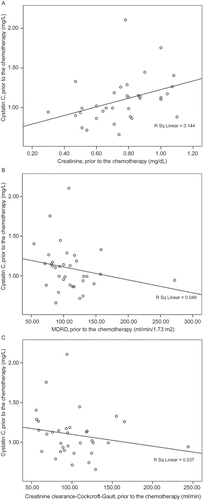
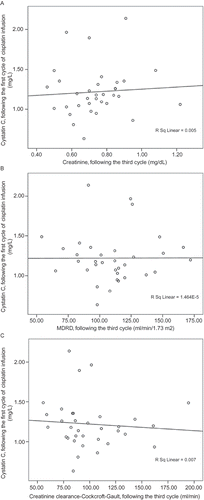
The mean serum creatinine level was 0.76 ± 0.20 mg/dL, and the mean serum cystatin C level was 1.10 ± 0.30 mg/L prior to the chemotherapy. The creatinine clearance calculated by the Cockcroft–Gault formulary was 102.5 ± 37.6 mL/min, and GFR calculated by MDRD was 109.9 ± 37.9 mL/min/1.73 m2. The mean cystatin C level was 1.22 ± 0.31 mg/L following the first cycle of cisplatin infusion. The mean creatinine level was 0.74 ± 0.16 mg/dL following the third cycle. The creatinine clearance was 100.3 ± 31.1 mL/min by the Cockcroft–Gault method, and GFR calculated by MDRD was 110.1 ± 26.8 mL/min/1.73 m2 ().
The cystatin C levels prior to the chemotherapy were 1.17 mg/L (95% CI: 1.00–1.33) in the patients with metastasis and 0.99 mg/L (95% CI: –0.91 to 1.08) in the patients without metastasis; there was no significant difference between them (p = 0.13). A statistically significant linear correlation was found between the serum cystatin C and creatinine levels prior to the chemotherapy (r = 0.42, p = 0.013) (). However, no significant correlation was found between MDRD and creatinine clearance–Cockcroft–Gault formulations (r = −0.28, p = 0.10 and r = −0.24, p = 0.17, respectively) ( and ). There were no correlations between the cystatin C levels and following parameters after cisplatin infusion: serum creatinine following the third course (r = 0.14, p = 0.41), MDRD (r = −0.05, p = 0.78), and creatinine clearance–Cockcroft–Gault (r = −0.19, p = 0.27) (–). The regression analysis demonstrated that the cystatin C levels prior to the chemotherapy or following the cisplatin infusion did not reflect the impairments in the renal function (the increase in serum creatinine and the decrease in GFR calculated by MDRD and creatinine clearance–Cockcroft–Gault) following the third course (p = 0.68, 0.98, and 0.82, respectively).
DISCUSSION
Even though the serum cystatin level was correlated with the serum creatinine level, it was not found to be an appropriate parameter in the prediction of chemotherapy-induced renal dysfunction.
Since the standard inulin clearance and 51Cr-EDTA methods cannot be performed at all centers, the high cost and the difficulty in the reproducibility of the scintigraphic methods resulted in the search for a novel marker. Although creatinine can be easily measured, it has a limitation in the detection of mild renal dysfunction unless the inulin clearance is lower than 50 mL/min/1.73 m2.Citation4 Because creatinine is not only filtrated but also secreted from the tubuli, GFR is overestimated with creatinine clearance.Citation5 Mathematical equations such as Cockcroft–Gault formulation, which is based on weight, age, gender, and creatinine level, is frequently used in daily practice without the need of collecting 24-h urine. In the study of Levey et al.,Citation22 it is reported that the MDRD formulation is a better GFR calculation method compared to the Cockcroft–Gault formulation. However, it has low sensitivity in moderate degree renal dysfunction. Some studies have reported that cystatin C can be used as an appropriate marker in monitoring the renal function, especially in children.Citation16,18,23 In fact, accurate and simple GFR measurement is crucial in elderly cancer patients. The results from the studies regarding adults are inconsistent.
In the study of Stabuc et al.,Citation8 it was reported that cystatin C was a better indicator in the determination of GFR compared to serum creatinine independent of metastasis and the cancer type. Likewise, Benohr et al.Citation9 found that the changes in the serum cystatin levels were correlated with the decrease in GFR, calculated by inulin clearance. On the other hand, in a study regarding ovarian cancer patients who received paclitaxel–cisplatin combination, no correlation was noted between cystatin C and the other GFR markers (serum creatinine level, Cockcroft–Gault, and MDRD formulations).Citation24 In the same study, significant correlations were found between the cystatin C levels and pretreatment tumor dimension and also with CA 125 levels. As a cysteine protease inhibitor, cystatin C is elevated in the presence of this tumor, which might explain why the cystatin C level does not reflect GFR. Some studies have reported high levels of cystatin C without any renal dysfunction in the patients with colorectal, head–neck, breast, and metastatic prostate cancers.Citation25–27 However, in a study regarding head and neck cancers, cystatin C was noted as a proper parameter for GFR measurement.Citation17 No correlation was found between the advanced tumor grade and cystatin C level in our study. Studies with larger patient groups will be necessary to prove that the serum cystatin C level is a proper method in determining the cisplatin-induced renal damage.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Patterson WP, Reams GP. Renal toxicities of chemotherapy. Semin Oncol. 1992;19:521–528.
- Weiss RB. Nephrotoxicity. In: DeVita VT, Hellman S, Rosenberg SA, eds. Principles and Practice of Oncology. Philadelphia: Lippincott-Raven Press; 1997:2796–2800.
- Mohanram A, Toto R. Measurement of kidney function. In: Pereira BJG, Sayegh MH, Blake PG, eds. Chronic Kidney Disease, Dialysis, and Transplantation. A Companion to Brenner and Rector's the Kidney. Philadelphia: Saunders; 2005:20–30.
- Perrone RD, Madias NE, Levy AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953.
- Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1990;130:461–470.
- Hergeth-Rosenthal S, Trabold S, Pietruck F, Holtmann M, Philipp T, Kribben A. Cystatin C: efficacy as screening test for reduced glomerular filtration rate. Am J Nephrol. 2000;20:97–102.
- Stabuc B, Vrhovec L, Stabuc-Silih M, Cizej TE. Improved prediction of decreased creatinine clearance by serum cystatin C: use in cancer patients before and during chemotherapy. Clin Chem. 2000;46:193–197.
- Benohr P, Grenz A, Hartmann JT, Müller GA, Blaschke S. Cystatin C—A marker for assessment of the glomerular filtration rate in patients with cisplatin chemotherapy. Kidney Blood Press Res. 2006;29:32–35.
- Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol. 1992;38:20–27.
- Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR—History, indications, and future research. Clin Biochem. 2005;38:1–8.
- Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47:2055–2059.
- Manetti L, Pardini E, Genovesi M, et al. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest. 2005;28:346–349.
- Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C—A new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–881.
- Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226.
- Bárdi E, Bobok I, Oláh AV, Oláh É, Kappelmayer J, Kiss C. Cystatin C is a suitable marker of glomerular function in children with cancer. Pediatr Nephrol. 2004;19:1145–1147.
- Bölke E, Schieren G, Gripp S, et al. Cystatin C—A fast and reliable biomarker for glomerular filtration rate in head and neck cancer patients. Strahlenther Onkol. 2011;187:191–201.
- Grönroos MH, Jahnukainen T, Irjala K, et al. Comparison of glomerular function tests in children with cancer. Pediatr Nephrol. 2008;23:797–803.
- Yano M, Hirai K, Naito Z, et al. Expression of cathepsin B and cystatin C in human breast cancer. Surg Today. 2001;31:385–389.
- Kos J, Werle T, Lah T, Brunner N. Cysteine proteinases and their inhibitors in extracellular fluids: marker for diagnosis and prognosis in cancer. Int J Biol Markers. 2000;15:84–89.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470.
- Lankisch P, Wessalowski R, Maisonneuve P, Haghgu M, Hermsen D, Kramm CM. Serum cystatin C is a suitable marker for routine monitoring of renal function in pediatric cancer patients, especially of very young age. Pediatr Blood Cancer. 2006;46:767–772.
- Bodnar L, Wcislo GB, Smoter M, et al. Cystatin C as a parameter of glomerular filtration rate in patients with ovarian cancer. Kidney Blood Press Res. 2010;33:360–367.
- Kos J, Krasovec M, Cimerman N, Nielsen HJ, Christensen IJ, Brünner N. Cysteine proteinase inhibitors stefin A, stefin B, and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res. 2000;6:505–511.
- Strojan P, Svetic B, Smid L, Kos J. Serum cystatin C in patients with head and neck carcinoma. Clin Chim Acta. 2004;344:155–161.
- Tumminello FM, Flandina C, Crescimanno M, Leto G. Circulating cathepsin K and cystatin C in patients with cancer-related bone disease: clinical and therapeutic implications. Biomed Pharmacother. 2008;62:130–135.