Abstract
Background: Association of methylenetetrahydrofolate reductase (MTHFR) 677C>T gene polymorphism with hyperhomocysteinemia, renal failure, and cardiovascular events is controversial. We investigated the relationship of MTHFR 677C>T polymorphisms with left ventricular hypertrophy (LVH) and renal insufficiency. Methods: Glomerular filtration rate (GFR) and left myocardial ventricular mass/m2 were assessed in 138 non-diabetic subjects (age, 50.93 ± 14.85 years; body mass index, 27.95 ± 5.98 kg/m2), 38 no-mutation wild MTHFR C677CC, 52 heterozygous MTHFR C677CT, and 48 homozygous MTHFR C677TT, all with adequate adherence to current international healthy dietary guidelines. Serum homocysteine, insulin resistance, high-sensitivity C-reactive-protein (hsCRP), parathyroid hormone, and renal artery resistive index (RRI) were challenged by odds ratio analysis and multiple linear regression models. Results: MTHFR 677C>T polymorphism showed higher GFR (73.8 ± 27.99 vs. 58.64 ± 29.95; p= 0.001) and lower renal failure odds (OR, 0.443; 95% confidence interval, 0.141–1.387) in comparison with wild MTHFR genotype. A favorable effect on GFR of MTHFR polymorphism is presented independently by the negative effects of LVH, increased intra-renal arterial resistance, and hyperparathyroidism; GFR is the significant predictive factor to LVH. Conclusions: Renal insufficiency in non-diabetic subjects is explained by interactions of MTHFR C677T polymorphism mutation with LVH, hsCRP, intact parathyroid hormone (iPTH), and RRI. Sign of these predictive effects is opposite: subjects with MTHFR 677C>T polymorphism have lower likelihood of renal insufficiency; differently, wild-type MTHFR genotype subjects have lower GFR and greater hsCRP, iPTH, RRI, and LVH.
INTRODUCTION
Mutations of the human methylenetetrahydrofolate reductase (MTHFR) gene have been associated with increased homocysteine (HCY) levels: this was suspected to increase risks of cardiovascular disease (CVD) in various populations, and particularly, in patients with renal disease, especially when in hemodialysis.Citation1 Epidemiological studies have identified hyperhomocysteinemia as an independent risk factor for coronary artery disease, at least in some ethnic groupCitation2 in which one of the more common MTHFR mutations (nucleotide 677C>T) results in a thermolabile enzyme,Citation3 lower folate levels, and an inefficient HCY metabolism.Citation4 Hyperhomocysteinemia appears independent from other risk factors,Citation5 and subsequent reports increased concerns around the related common genetic polymorphism.Citation6 Nonetheless, earlier studies already challenged this conceptCitation7 and outlined that this mutation is not associated with premature death, since its prevalence in the elderly is not lower than in the young.Citation8 In other contexts, actually, the presence of the allele 677T of the MTHFR gene was the best explaining protective factor against cervical carcinogenesisCitation9 and for colonic cancer,Citation10–Citation12 seemingly associated with longer and healthier survival.Citation13 Nonetheless, according to other studies, MTHFR 677TT homozygous and systolic blood pressure independently influence intima-media thickness,Citation14 as other non-genetic markersCitation15 and nutritional conditions do.Citation16 Also mild–moderate renal impairment is associated with mortality, increased left ventricular myocardial mass (LVMM),Citation17 lower ejection fraction, and increased E/A ratio at echocardiography.Citation18 Insulin resistance accounts significantly for left ventricular (LV) mass increase in normotensive individuals.Citation19 A linear relationship between left myocardial ventricular mass/m2 (LVMMi) versus cardiovascular events, a J-shape relationship between LVMMi versus all-cause death,Citation20 and NT-proBNP increase in patients with left ventricular hypertrophy (LVH) suggest a common pathway, through the increase of measured myocardial mass, toward cardiac insufficiency.Citation21 Relevance of hyperhomocysteinemia stems from many considerations. Among them, in general population with no history of CVD, concentrations of HCY alone could accurately identify those at high risk of cardiovascular mortality, whereas classic risk factors included in the Framingham risk score do not,Citation22 suggesting the need of intervention.Citation23 MTHFR polymorphismsCitation24,Citation25 seemingly intervene, not only inducing hyperhomocysteinemia, within a cluster of different and even-interrelated conditions, diseases, and indexes. Dietary profiles are the background of any adequate nutrient intakes and particularly of a normal B vitamin intake and availability: they can be modified by conditions impairing renal function.Citation26 MTHFR gene–Mediterranean Diet interactions on HCY metabolism was reported: this dietary profile may reduce HCY concentrations and consequently influence coronary risk in genetically high-risk individuals, by quality and proportion of nutrients.Citation27 The accompanying body size increase is not invariably detrimental since, actually, patients with established chronic disease benefit of large body size.Citation28 This finding, defined the obesity paradox, is shared over a variety of cardiovascular, pulmonary, and renal diseases: it challenges the concept about differences for optimal body size in health and disease.Citation29 The cornerstone is how several metabolic factors affect renal circulation and, as a consequence, renal function. The increase of intra-renal artery resistance, measured by renal artery resistive index (RRI), affects the natural history of atherosclerosis and arterial hypertension, which was found to correlate with LVH and carotid intimal thickening,Citation29 with cardiovascular risk score and impaired renal outcome and death.Citation30 Also endocrine factors are very relevant: among them, parathyroid hormone (PTH) intervenes in several mechanisms of disease progression, including LVH,Citation31 impairment of renal function,Citation32 and increase of intra-renal arterial resistance.Citation32–Citation34 The aim of this study was to investigate relationship of MTHFR 677C>T polymorphisms with glomerular filtration rate (GFR) and with LVMM, dietary profile, high-sensitivity C-reactive-protein (hsCRP), intact parathyroid hormone (iPTH), insulin resistance, and RRI in non-diabetic subjects.
PATIENTS AND METHODS
We studied 138 Italian Caucasian subjects, aged 50.93 ± 14.85 years [body mass index (BMI) 27.95 ± 5.98 kg/m2]: 38 MTHFR C677CC (wild genotype), 52 heterozygous MTHFR C677CT, and 48 homozygous MTHFR C677TT (tremolabile polymorphism) subjects. They were referred to the Internal Medicine Day hospital for clinical assessment and lifestyle–nutritional counseling. Preliminary exclusion criteria were as follows: (1) all patients with clinical/echographic signs of congestive heart failure, malignancies, severe chronic liver disease, apart from the lone finding of bright liver; (2) patients with any history of diabetes mellitus, established by a fasting glucose level ≥ 126 mg/dL or HbA1c ≥ 6.5%, or those under treatment for other diseases, apart from well-controlled arterial hypertension; and (3) individuals who were extremely obese (class III: BMI ≥ 40) and underweight subjects (BMI < 18.5). Subsequent exclusion criteria were the following: all patients with acute or chronic infectious disease, a history of alcohol abuse (exceeding 20 g/d), severe renal insufficiency (GFR < 30 mL/min/1.73 m2 or detection of proteinuria), thyroid disease, polycystic ovary syndrome, and steroid use. Arterial hypertension, defined as >140 mmHg of systolic and >90 mmHg of diastolic blood pressure, was not an exclusion criterion, provided that an adequately stable normal blood pressure was achieved and maintained. Throughout the study, no patient was eligible if recently treated with statins, metformin, or other drugs with known effects on insulin resistance. Patients were managed by Mediterranean Diet and lifestyle counseling, including physical exercise prescription. Mediterranean Diet Adherence Profile was assessed as Adherence to Mediterranean Diet Score (AMDS) on the basis of a 1 week recall computerized questionnaire; this is a premise to personalized Mediterranean Diet prescriptions, with daily recommendations also derived from the specific software used (Dietosystem, Milan, Italy).Citation32 Physical activity increase and smoking withdrawal active counseling were also provided.Citation35 The criteria used for delivering the Mediterranean Diet Score included few modifications Citation35–Citation37 in comparison with the original report.Citation38–Citation40 Patients’ assessment was performed at the end of the first month of observation after the initial enrollment, which included the prescription and planning of dietary and lifestyle changes. Suggestions and advice on individual “healthy” food purchase, storage, and cooking were provided; reliable feedback and evidence of patients’ adherence were obtained by scheduled dietician’s interviews. Physical activity was also encouraged in the form of walking using the “10,000 steps a day” suggestion, maintaining, if present, the current leisure or sport habits. A portable electronic pedometer (step counter) was also given as a monitoring and as a motivation toolCitation41 to enhance and maintain daily physical activity increase. Routine laboratory tests included virus hepatitis (hepatitis A virus, HBV, and HCV) and cancer biomarkers (AFP, CEA, Ca125, and Ca15-3), thyroid hormones (TSH), aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, ferritin, total protein, and albumin. Human insulin and folic acid were assayed using Immulite 2000 Analyzer, by a solid-phase 2-site chemiluminescent immunometric assay. hsCRP concentrations were assayed by a standard detection limit of 0.175 mg/L (CardioPhase high-sensitivity hsCRP method, Siemens Medical System, Milan, Italy). HCY and B12 vitamin assay in the blood were performed by ADVIA Centaur® XP Immunoassay (Siemens Medical System, Milan, Italy).Citation42 iPTH and NT-proBNP (IMMULITE® 2000, Siemens Medical System, Milan, Italy) were assessed by a solid-phase two-site chemiluminescent immunometric assay. PTH values considered normal were <70 pg/mL for subjects without severe renal insufficiency.Citation43 Body weight (BW) was measured in light clothing, without shoes, in kilograms, and height (H) was measured in meters, using a scale-integrated stadiometer. BMI was calculated as BW/H2, and patients were categorized as normal weight (<25.0 kg/m2), overweight (≥25.0 and ≤29.9 kg/m2), and obese (≥30.0 kg/m2). Insulin resistance was assessed by the homeostasis model-insulin resistance index (HOMA) according to the following formulas: fasting insulin value × fasting blood sugar level/405. The HOMA threshold for insulin resistance is conventionally considered >1.7, according to the likelihood ratios for 11-year CVD prediction.Citation44 The waist-to-hip (W/H) ratio was assessed in all patients. Ultrasound (US) examinations were performed by echographists unaware of laboratory details at the time of the procedure. An echo-color-doppler machine (Siemens Acuson S2000™, Siemens AG, Muenchen Germany), high resolution, with real-time sectional scan transducers was used. Renal color Doppler echography is performed assessing intra-parenchymal RRI (peak systolic velocity—end-diastolic velocity/peak systolic velocity).Citation28,Citation45 First measurement is the size of the left and right kidney. For orientation purposes, perfusion in the whole of the left and right kidneys is then checked using color Doppler ultrasonography, and the main trunk of the renal artery is displayed. Three measurements for each kidney are taken by pulsed Doppler within 5 min in the vicinity of the interlobar artery. RRI is calculated as the average value of all measurements taken. RRI threshold to define higher RRI measurements is defined by the 75th percentile derived by measurements of all eligible patients.Citation46 Echocardiographic studies were performed with two-dimensional guided M-mode echocardiography according to methods established by the American Society of Echocardiography (ASE)Citation47–Citation49 with transducer frequencies appropriate for body size. Siemens Acuson S2000™, Siemens AG, Munich, Germany, or a GE echo-color-doppler device (GE Logiq7 Expert US, manufactured by GE Medical Systems, Milwaukee, Wisconsin), high resolution, with real-time sectional scan transducers was used. An average of two echocardiographic measurements was taken, and the cardiologist reading them was blinded to the clinical information of the patient. Measurements were obtained for LV end-diastolic and end-systolic dimension, septal wall thickness, and posterior wall thickness in diastole. LVM was calculated with the method of Devereux et al.Citation50 and indexed by dividing by body surface area/m2. All the exams were stored on digital media for subsequent analysis. LV diameters and wall thickness were measured according the ASE guidelines,Citation47 and LV ejection fraction (LVEF) was computed using the modified Simpson’s formula. LVEF was considered abnormal if <50%. GFR is assessed as estimated GFR by the modification of diet in renal disease (MDRD) formula in mL/min per 1.73 m2, according to the Clinical Practice Guidelines for Chronic Kidney Disease KDOQI.Citation42 Genotypes of the MTHFR C677T and A1298C polymorphisms were detected by polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP). DNA was extracted from peripheral blood by a commercially available DNA isolation method (QIAamp DNA Blood Mini Kit QIAGEN, Milan, Italy). Restriction enzyme analysis of amplified product (PCR-RFLP) analysis was carried out for direct genotypes detection of SNPs, C667T (rs1801133), and A1298C (rs1801131). PCR products were obtained using specific primers (NCBI Reference Sequence: NG_013351.1): C667T (F5′-GTCCCTGTGGTCTCTTCATCC-3′/R5′-GGTGGCCAAGCAACGCTGTG-3′); A1298C (F5′-CTTCTACCTGAAGAGCAAGTC-3′/R5′-CACATGTCCACAGCATGGAC-3′). Both amplicons were successively digested by HinfI and MboII restriction enzymes for C667T and A1298C, respectively, and DNA fragment was visualized in a 4% agarose gel stained with SYBR safe (Life Technologies Italia, Monza, Italy); electrophoresis pattern was used to determined MTHFR genotypes.Citation51 Within all patients referred for clinical assessment and included in the MTHFR genetic diagnostic panel, A1298C homozygous, heterozygous, and compound MTHFR 677CT heterozygous polymorphism subjects (n = 137) were excluded from the analysis of data. Informed consent was obtained from each patient, relatively also to the use of genetic information, and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Statistical Analysis
The fit to the Hardy–Weinberg equilibrium was analyzed. The distributions of MTHFR alleles and genotypes in studied group were checked by χ2 test or Fisher’s exact test. Descriptive results of continuous variables are expressed as averages (±SD). Student’s t-test was used to assess the difference in averages between subjects with MTHFR heterozygous and homozygous polymorphism versus wild genotype subjects. Two-sided p-value <0.05 was considered statistically significant. Higher quartiles of age, homocysteine, iPTH, RRI, hsCRP, and other continue measures were defined; thereafter, the associations of older age, higher hsCRP, iPTH, RRI, LVH (LVMMi ≥150 g/m2 in men, ≥120 g/m2 in womenCitation52), and MTHFR C677T polymorphisms were assessed as odds ratios (ORs) to renal insufficiency (GFR ≤ 90 mL/min/m2) with 95% confidence intervals (CI). A multiple linear regression (MLR) model, age-balanced, challenges MTHFR C677T polymorphism toward GFR and includes AMDS, RRI, HOMA, HCY, hsCRP, iPTH, B12 vitamin, folic acid, and LVMMi as predictive variants. An analogous MLR model, age-balanced, challenges MTHFR C677T polymorphism toward LVMM/m2 (LVMMi) and includes AMDS, RRI, HOMA, HCY, hsCRP, iPTH, B12 vitamin, folic acid, and GFR as predictive variants. All analyses were performed using SPSS 18.0 for Windows (SPSS, Chicago, IL, USA), Power analysis by G*Power 3.1 and graphs by GraphPad-Prism.
RESULTS
The differences in averages between patients with MTHFR 677C>T heterozygous and homozygous polymorphism versus wild genotype subjects are shown in . GFR is significantly higher in the MTHFR 677C>T polymorphism group versus wild genotype subjects (73.68 ± 27.89 vs. 58.27 ± 29.69; p: 0.005); HCY (21.27 ± 5.02 vs. 19.37 ± 4.27; p: 0.040), and LDL cholesterol (129.85 ± 42.25 vs. 112.97 ± 37.24; p: 0.032) are slightly higher in the MTHFR 677C>T polymorphism group versus wild genotype subjects. A significant linear correlation of GFR versus LVMMi (r = −0.388; p < 0.0001) is observed. Significant inverse correlation of age versus GFR (r = −0.501; p < 0.0001) and direct correlations of age versus RRI (r = 0.491; p < 0.0001) and versus LVMMi (r = 0.275; p < 0.001) are observed. iPTH shows significant inverse correlation versus GFR (r = −0.366; p < 0.0001), whereas a direct trend of iPTH is observed versus RRI (r = 0.292; p < 0.001) and versus LVMMi (r = 0.162; p < 0.05). No significant correlation is observed for both hsCRP and insulin resistance (HOMA) versus GFR, LVMMi, and RRI.
Table 1. Characteristic of study population and differences between MTHFR polymorphism and control group.
By MLR, age-balanced, GFR is the only factor that explains significantly 18.0% of the variance to LVH, assessed as LVMMi, in the MLR model (, top). A predictive effect of MTHFR 677C>T polymorphism versus lower GFR is significantly displayed, along with the opposite unfavorable effects of higher PTH, LVMMi, and RRI; these last are all conditions for lower GFR and, together, explain 45.2% of the variance toward GFR (, bottom).
Table 2. Multiple linear regression predictive model for LVMM/m2 and GFR.
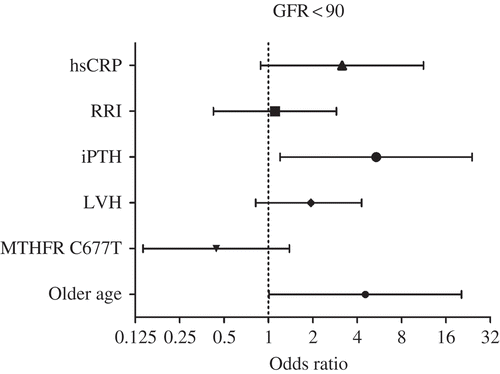
By OR (), higher iPTH (OR, 5.377; 95% CI, 1.202–24.051), greater hsCRP (OR, 3.156; 95% CI, 0.885–11.255), older age (OR, 4.543; 95% CI, 1.012–20.397), and LVH (OR, 1.942; 95% CI, 0.819–4.293) are associated significantly with renal insufficiency; MTHFR 677C>T polymorphism is associated significantly with lower odds of renal insufficiency (OR, 0.443; 95% CI, 0.141–1.387).
Figure 1. Higher concentration of parathyroid hormone (iPTH) (OR, 5.377; 95% CI, 1.202–24.051), greater levels of high-sensitivity C-reactive protein (hsCRP) (OR, 3.156; 95% CI, 0.885–11.255), older age (OR, 4.543; 95% CI, 1.012–20.397), and left ventricular hypertrophy (LVH) (OR, 1.942; 95% CI, 0.819–4.293) are associated significantly with renal insufficiency; MTHFR 677C>T polymorphism is associated significantly with lower odds of renal insufficiency (OR, 0.443; 95% CI, 0.141–1.387).
DISCUSSION
According to our results, renal insufficiency in adult non-diabetic subjects is explained by the interaction of MTHFR C677T polymorphism with other independent factors, that is, iPTH, LVMMi, and RRI. Myocardial LVH (assessed by LVMMi) has a single significant predictor, that is, lower GFR, while the other factors considered by our investigation, including MTHFR mutation, do not show this effect: our finding, age-independent, confirms the close relationship of GFR and LVMM and their parallel progression.Citation19,Citation53 Mild–moderate renal insufficiency, assessed by GFR, is considered a comprehensive expression of the effects of multiple factors on renal function outcome,Citation53 while LVH, assessed as LVMM/m2, is used as a broad measure of the lasting effects of different mechanisms on myocardial mass. Mild–moderate renal insufficiency is associated with increased LVMMi.Citation17 LVH is a broad measure of the lasting effects of different mechanisms on myocardial anatomy and function.Citation19 We do not confirm the independent increased risk to LVH reported in association with greater CRP,Citation54 increased intra-renal arterial resistance, as assessed by RRI,Citation32 and increased serum iPTH.Citation55 Nonetheless, lower GFR is well recognized as a factor related with greater CRP,vCitation56,Citation57 increased intra-renal arterial resistance, as assessed by RRI,Citation58,Citation59 and increased serum iPTH.Citation32 MTHFR C677T polymorphism has predictive effects on GFR: subjects with MTHFR C677T polymorphism have a lower likelihood of renal insufficiency in comparison with subjects with the wild MTHFR genotype. This result is not surprising since this mutation is associated with a protective effect versus very prevalent cancer diseases9–12 and is not disadvantageous for longevity.Citation13 HCY is settled as a putative risk factor for CVD,Citation60 and mechanisms for glomerular injury and progression of renal insufficiency are envisaged.Citation61 Nonetheless, related genetic background, such as MTHFR mutation, cannot be necessarily detrimental. Insulin resistance and obesity are recognized as LV mass determinants independent of blood pressureCitation19; we failed to confirm this relationship, and probably the exclusion of diabetic patients is the reason of this result. Relationships of diabetes, insulin resistance, and subclinical hyperinsulinemia/hyperglycemia with cardiac structure and function are recognized: both were consistently implicated in concentric LV remodelingCitation62,Citation63 and in development of chronic kidney disease with rapid decline in renal function.Citation64 Although high-dose folic acid would slow the progression of atherosclerosis and reduce cardiovascular events in patients with chronic renal failure, counteracting effects of hyperhomocysteinemia is still debated and not demonstrated.Citation65 Differently, there is a good consistency of data that establish renal involvement and LVH as novel risk factors for morbidity and mortality in diabetes mellitus.Citation66 Cardiac remodeling, also with increase of LVMM, is a premise toward the development of heart insufficiency,Citation67 which could be redefined as also encompassing serological biomarkers.Citation68 The favorable relevance of adherence to healthier nutritional profile and lifestyle changes is well established and warranted in cardiac diseaseCitation69,Citation70 and also, by more recent contributions, in renal disease.Citation71 In earlier studies,Citation72,Citation73 relationship of MTHFR C677T mutation with renal and cardiac involvement was associated with precocious target organ damage. Actually, in younger subjectsCitation74 and in other reports,Citation75 homozygosity for the C677T mutation is not unequivocally associated with increased risk for CVD, irrespective of folate intake. This is confirmed by a recent extensive epidemiological study, in which despite lower serum folate and higher homocysteine, MTHFR 677TT genotype, used as a proxy for lifelong high blood HCY concentrations, is associated with a significantly lower risk of CVD mortality.Citation76 Hyperhomocysteinemia is common in patients with severe heart failure, and plasma homocysteine levels are uniformly elevated regardless of the etiology of heart failure. Elevated plasma homocysteine levels are likely a consequence of heart failure–related renal insufficiency.Citation77 Moreover, high HCY levels in patients with end-stage renal disease were not associated with incidence of vascular access thrombosis.Citation78 In our study, MTHFR C677T mutation occurs in a population that has still a relatively low prevalence of cardiovascularCitation15 and renal disease.Citation71 It is possible that this polymorphism, even associated with greater LVMMi, could have maintained its persistence in human populations by a heterozygosis-mutant advantage mechanism exerted over more critical conditions, including the occurrence of renal insufficiency. All-cause and coronary heart disease death rates are low in cohorts with greater adherence to Mediterranean Diet.Citation15
CONCLUSION
MTHFR 677C>T gene polymorphism could have a protective role on renal function in non-diabetic patients without hyperhomocysteinemia and adequate alimentary regimen.
ACKNOWLEDGMENT
These results were presented in part at the American Society for Nutrition 76th Annual Meeting held in San Diego, CA, April 21–25, 2012, and published as abstract on FASEB J (2012) 26: lb328
Declaration of interest: No conflict of interests that might lead to bias is declared by any of the Authors.
No grant or financial support, Public, Institutional or Private, was received by any Author for this or any related research, study, or activity.
REFERENCES
- Fung MM, Salem RM, Lipkowitz MS, Methylenetetrahydrofolate reductase (MTHFR) polymorphism A1298C (Glu429Ala) predicts decline in renal function over time in the African-American Study of Kidney Disease and Hypertension (AASK) Trial and Veterans Affairs Hypertension Cohort (VAHC). Nephrol Dial Transplant. 2012;27:197–205.
- Morita H, Taguchi J, Kurihara H, A common mutation in methylenetetrahydrofolate reductase gene is not a major risk of coronary artery disease or myocardial infarction. Atherosclerosis. 1997;128:107–112.
- Minamino T, Ohno M, Yamaoki K, Genetic polymorphism of 5,10-methylenetetrahydrofolate reductase (MTHFR) as a risk factor for coronary artery disease. Circulation. 1997;95:2032–2036.
- van Bockxmeer FM, Mamotte CD, Vasikaran SD, Taylor RR. Methylenetetrahydrofolate reductase gene and coronary artery disease. Circulation. 1997;95:21–23.
- Deloughery TG, Evans A, Sadeghi A, Common mutation in methylenetetrahydrofolate reductase. Correlation with homocysteine metabolism and late-onset vascular disease. Circulation. 1996;94:3074–3078.
- Kang SS, Passen EL, Ruggie N, Wong PW, Sora H. Thermolabile defect of methylenetetrahydrofolate reductase in coronary artery disease. Circulation. 1993;88(4 Pt 1):1463–1469.
- Gülec S, Aras O, Akar E, Tutar E, Methylene-tetrahydro-folate reductase gene polymorphism and risk of premature myocardial infarction. Clin Cardiol. 2001;24:281–284.
- Brattström L, Zhang Y, Hurtig M, A common methylenetetrahydrofolate reductase gene mutation and longevity. Atherosclerosis. 1998;141:315–319.
- Agodi A, Barchitta M, Cipresso R, Distribution of p53, GST, and MTHFR polymorphisms and risk of cervical intraepithelial lesions in Sicily. Int J Gynecol Cancer. 2010;20:141–146.
- Chen J, Giovannucci E, Kelsey K, A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res. 1996;56:4862–4864.
- Martínez ME, Thompson P, Jacobs ET, Dietary factors and biomarkers involved in the methylenetetrahydrofolate reductase genotype-colorectal adenoma pathway. Gastroenterology. 2006;131:1706–1716.
- Lee JE, Wei EK, Fuchs CS, Plasma folate, methylenetetrahydrofolate reductase (MTHFR), and colorectal cancer risk in three large nested case-control studies. Cancer Causes Control. 2012;23:537–545.
- Rea IM, McMaster D, Woodside JV, Community-living nonagenarians in Northern Ireland have lower plasma homocysteine but similar methylenetetrahydrofolate reductase thermolabile genotype prevalence compared to 70-89-year-old subjects. Atherosclerosis. 2000;149:207–214.
- Ravera M, Viazzi F, Berruti V, 5,10-Methylenetetrahydrofolate reductase polymorphism and early organ damage in primary hypertension. Am J Hypertens. 2001;14(4 Pt 1):371–376.
- Dedoussis GV, Panagiotakos DB, Pitsavos C, ATTICA Study Group. An association between the methylenetetrahydrofolate reductase (MTHFR) C677T mutation and inflammation markers related to cardiovascular disease. Int J Cardiol. 2005;100:409–414.
- Trovato GM, Pirri C, Martines GF, Trovato F, Catalano D. Coffee, nutritional status, and renal artery resistive index. Ren Fail. 2010;32:1137–1147.
- Greaves K, Chen R, Ge L, Mild to moderate renal impairment is associated with increased left ventricular mass. Int J Cardiol. 2008;124:384–386.
- Hsieh MC, Su HM, Wang SY, Significant correlation between left ventricular systolic and diastolic dysfunction and decreased glomerular filtration rate. Ren Fail. 2011;33:977–982.
- Rodrigues SL, Angelo LC, Pereira AC, Krieger JE, Mill JG. Determinants of left ventricular mass and presence of metabolic risk factors in normotensive individuals. Int J Cardiol. 2009;135:323–330.
- Lai CL, Chien KL, Hsu HC, Su TC, Chen MF, Lee YT. Left ventricular mass and risk of cardiovascular events and all-cause death among ethnic Chinese – the Chin-Shan Community Cardiovascular Cohort study. Int J Cardiol. 2011;149:347–352.
- Rivera Otero JM, Taléns-Visconti R, Salvador A, Grupo de Disfunción VI, Comunidad Valenciana. Ventricular hypertrophy increases NT-proBNP in subjects with and without hypertension. Int J Cardiol. 2004;96:265–271.
- de Ruijter W, Westendorp RG, Assendelft WJ, Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083–a3090.
- Wald DS, Law M, Morris JK. The dose-response relation between serum homocysteine and cardiovascular disease: implications for treatment and screening. Eur J Cardiovasc Prev Rehabil. 2004;11:250–253.
- Brattström L, Wilcken DE. Homocysteine and cardiovascular disease: cause or effect? Am J Clin Nutr. 2000;72:315–323.
- Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am J Clin Nutr. 2000;72:324–332.
- Kopple JD. The phenomenon of altered risk factor patterns or reverse epidemiology in persons with advanced chronic kidney failure. Am J Clin Nutr. 2005;81:1257–1266.
- Dedoussis GV, Panagiotakos DB, Chrysohoou C, Effect of interaction between adherence to a Mediterranean Diet and the methylenetetrahydrofolate reductase 677C–>T mutation on homocysteine concentrations in healthy adults: the ATTICA Study. Am J Clin Nutr. 2004;80:849–854.
- Doehner W, Clark A, Anker SD. The obesity paradox: weighing the benefit. Eur Heart J. 2010;31:146–148.
- Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol. 2003;180:885–892.
- Heine GH, Reichart B, Ulrich C, Köhler H, Girndt M. Do ultrasound renal resistance indices reflect systemic rather than renal vascular damage in chronic kidney disease? Nephrol Dial Transplant. 2007;22:163–170.
- Saleh FN, Schirmer H, Sundsfjord J, Jorde R. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J. 2003;24:2054–2060.
- Anderson JL, Vanwoerkom RC, Horne BD, . Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: dependent or independent risk factors? Am Heart J. 2011;162:331–339.
- Trovato GM, Martines GF, Trovato FM, Pirri C, Pace P, Catalano D. Renal resistive index and parathyroid hormone relationship with renal function in nondiabetic patients. Endocr Res. 2012;37:47–58.
- Bell DS. Insulin resistance. An often unrecognized problem accompanying chronic medical disorders. Postgrad Med. 1993;93(99-103):106–107.
- Pitsavos C, Panagiotakos DB, Chrysohoou C, Stefanadis C. Epidemiology of cardiovascular risk factors in Greece: aims, design and baseline characteristics of the ATTICA study. BMC Publ Health. 2003;3:32–40.
- Catalano D, Trovato GM, Martines GF, Randazzo M, Tonzuso A. Bright liver, body composition and insulin resistance changes with nutritional intervention: a follow-up study. Liver Int. 2008;28:1280–1287.
- Dai J, Jones DP, Goldberg J, Association between adherence to the Mediterranean Diet and oxidative stress. Am J Clin Nutr. 2008;88:1364–1370.
- Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Diet and overall survival in elderly people. BMJ. 1995;31:1457–1460.
- Trichopoulou A, Vasilopoulou E. Mediterranean Diet and longevity. Br J Nutr. 2000;84(Suppl 2):S205–S209.
- Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean Diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608.
- Bravata DM, Smith-Spangler C, Sundaram V, Using pedometers to increase physical activity and improve health: a systematic review. J Am Med Assoc. 2007;298:2296–2304.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):S1–S201.
- La’ulu SL, Rawlins ML, Pfeiffer CM, Zhang M, Roberts WL. Performance characteristics of six homocysteine assays. Am J Clin Pathol. 2008;130:969–975.
- Rutter MK, Wilson PW, Sullivan LM, Fox CS, D’Agostino RBSr, Meigs JB. Use of alternative thresholds defining insulin resistance to predict incident type 2 diabetes mellitus and cardiovascular disease. Circulation. 2008;117:1003–1009.
- Trovato GM, Catalano D, Sciacchitano G, Zuccalà G, Iannetti E. Resistive index of renal artery and blood pressure in postmenopausal women. Maturitas. 2002;41:223–230.
- Trovato GM, Pirri C, Martines GF, Tonzuso A, Trovato F, Catalano D. Lifestyle interventions, insulin resistance, and renal artery stiffness in essential hypertension. Clin Exp Hypertens. 2010;32:262–269.
- Gardin JM, Adams DB, Douglas PS, American Society of Echocardiography. Recommendations for a standardized report for adult transthoracic echocardiography: a report from the American Society of chocardiography’s Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. J Am Soc Echocardiogr. 2002;15:275–290.
- Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083.
- Woythaler JN, Singer SL, Kwan OL, Accuracy of echocardiography versus electrocardiography in detecting left ventricular hypertrophy: comparison with postmortem mass measurements. J Am Coll Cardiol. 1983;2:305–311.
- Devereux RB, Alonso DR, Lutas EM, Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458.
- Guéant-Rodriguez RM, Guéant JL, Debard R, Prevalence of methylenetetrahydrofolate reductase 677T and 1298C alleles and folate status: a comparative study in Mexican, West African, and European populations. Am J Clin Nutr. 2006;83:701–707.
- Foppa M, Duncan BB, Rohde LE. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound. 2005;3:17–30.
- Smith GL, Lichtman JH, Bracken MB, Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996.
- Iwashima Y, Horio T, Kamide K, Rakugi H, Ogihara T, Kawano Y. C-reactive protein, left ventricular mass index, and risk of cardiovascular disease in essential hypertension. Hypertens Res. 2007;30:1177–1185.
- Andersson P, Rydberg E, Willenheimer R. Primary hyperparathyroidism and heart disease; a review. Eur Heart J. 2004;25:1776–1787.
- Fox ER, Benjamin EJ, Sarpong DF, The relation of C–reactive protein to chronic kidney disease in African Americans: the Jackson Heart Study. BMC Nephrol. 2010;11:1–7.
- Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–821.
- Petersen LJ, Petersen JR, Talleruphuus U, Ladefoged SD, Mehlsen J, Jensen HA. The pulsatility index and the resistive index in renal arteries. Associations with long-term progression in chronic renal failure. Nephrol Dial Transplant. 1997;12: 1376–1380.
- Catalano D, Trovato GM, Martines GF, Pirri C, Trovato FM. Renal function and severity of bright liver. Relationship with insulin resistance, intrarenal resistive index, and glomerular filtration rate. Hepatol Int. 2011;5:822–829.
- Maurer M, Burri S, de Marchi S, Plasma homocysteine and cardiovascular risk in heart failure with and without cardiorenal syndrome. Int J Cardiol. 2010;141:32–38.
- Yi F, Li PL. Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am J Nephrol. 2008;28:254–264.
- Rutter MK, Parise H, Benjamin EJ, Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454.
- Velagaleti RS, Gona P, Chuang ML, Relations of insulin resistance and glycemic abnormalities to cardiovascular magnetic resonance measures of cardiac structure and function: the Framingham Heart Study. Circ Cardiovasc Imaging. 2010;3:257–263.
- Cheng HT, Huang JW, Chiang CK, Yen CJ, Hung KY, Wu KD. Metabolic syndrome and insulin resistance as risk factors for development of chronic kidney disease and rapid decline in renal function in elderly. J Clin Endocrinol Metab. 2012;97:1268–1276.
- Zoungas S, McGrath BP, Branley P, Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47:1108–1116.
- Boner G. Renal involvement and left ventricular hypertrophy are novel risk factors for morbidity and mortality in diabetes mellitus. Diabetes Metab Res Rev. 2011;27:425–429.
- Florea VG, Mareyev VY, Samko AN, Orlova IA, Coats AJ, Belenkov YN. Left ventricular remodelling: common process in patients with different primary myocardial disorders. Int J Cardiol. 1999;68:281–287.
- Thomas MD, Fox KF, Coats AJ. Redefining heart failure. Int J Cardiol. 2006;112:139–141.
- de Lorgeril M, Salen P. Mediterranean Diet in secondary prevention of CHD. Public Health Nutr. 2011;14:2333–2337.
- Coats AJ. Advances in the non-drug, non-surgical, non-device management of chronic heart failure. Int J Cardiol. 2005;100:1–4.
- Chrysohoou C, Panagiotakos DB, Pitsavos C, Adherence to the Mediterranean Diet is associated with renal function among healthy adults: the ATTICA study. J Ren Nutr. 2010;20:176–184.
- Pereira AC, Miyakawa AA, Lopes NH, Dynamic regulation of MTHFR mRNA expression and C677T genotype modulate mortality in coronary artery disease patients after revascularization. Thromb Res. 2007;121:25–32.
- Kalina A, Czeizel AE. The methylenetetrahydrofolate reductase gene polymorphism (C677T) is associated with increased cardiovascular mortality in Hungary. Int J Cardiol. 2004;97:333–334.
- Collings A, Raitakari OT, Juonala M, Associations of methylenetetrahydrofolate reductase C677T polymorphism with markers of subclinical atherosclerosis: the cardiovascular risk in Young Finns Study. Scand J Clin Lab Invest. 2008;68:22–30.
- Schmitz C, Lindpaintner K, Verhoef P, Gaziano JM, Buring J. Genetic polymorphism of methylenetetrahydrofolate reductase and myocardial infarction: a case-control study. Circulation. 1996;94:1812–1814.
- Yang Q, Bailey L, Clarke R, Prospective study of methylenetetrahydrofolate reductase (MTHFR) variant C677T and risk of all-cause and cardiovascular disease mortality among 6000 US adults. Am J Clin Nutr. 2012;95:1245–1253.
- Schofield RS, Wessel TR, Walker TC, Cleeton TS, Hill JA, Aranda JM Jr. Hyper-homocysteinemia in patients with heart failure referred for cardiac transplantation: preliminary observations. Clin Cardiol. 2003;26:407–410.
- Bowden RG, Wyatt FB, Wilson R, Wilborn C, Gentile M. Homocysteine and vascular access thrombosis in a cohort of end-stage renal disease patients. Ren Fail. 2004;26:709–714.
APPENDIX
The traditional Mediterranean diet prescribed is characterized by a high intake of vegetables, legumes, fruits and nuts, and cereals, a high intake of olive oil, a low or no intake of saturated lipids, a moderately high intake of fish, a low-to-moderate intake of dairy products (mostly in the form of cheese or yogurt), and a low intake of meat and poultry. The subjects reported their daily or weekly average intake of several food items that they consumed during the last year. Then, the frequency of consumption was quantified approximately in terms of the number of times a month this food was consumed. Thus, daily consumption was multiplied by 30 and weekly consumption was multiplied by 4: a value of 0 was assigned to food items rarely or never consumed;Citation1 daily consumption of nonrefined cereals and products (e.g., whole-grain bread, pasta, brown rice, etc.), fruits (4–6 servings/day), vegetables (2–3 servings/day), olive oil (as the main added lipid), and non-fat or low-fat dairy products (1–2 servings/day); Citation2 weekly consumption of fish, poultry, potatoes, olives, pulses, and nuts (4–6 servings/week) and more rarely eggs and sweets (1–3 servings/week) and monthly consumption of red meat and meat products (4–5 servings/month). According to the previous dietary pattern and the reported monthly frequency consumption of these food groups, we calculated each participant’s diet score, which assessed adherence to the Mediterranean diet (range 0–55).
Adherence to Mediterranean Diet Score criteria can be summarized as follows:
Mediterranean food (I Pasta and rice; II whole-grain bread, brown rice, legumes; III Fruit; IV Green vegetables; V Fish, poultry, No-fat or low-fat dairy products; VI olive oil) had assigned, each group of food, the following scores: 0 = no consumption; a score of 1 = 1–4 times/month; 2 = 5–8 times/month; 3 = 9–11 times/month; 4 = 12–14 times/month; and 5 = more than 14 times/month. “Westernized food”: (VII Red meat; VIII Dairy products-butter; IX Potatoes and eggs; X Cakes) opposite scores were assigned, each group of food, the following scores: 5 = 0–4 monthly consumption; score 4 = 5–8 monthly consumption; 3 = 9–12 monthly consumption; 2 = 13–16 monthly consumption; 1 = 17–20 monthly consumption; 0 = more than 20 monthly consumptions). XI Wine and alcoholics (on average daily base): (0–10 g of alcoholics from Red Wine for women score 5; 0–20 g of alcoholics from Red Wine for men score 5); each increment of 10 g, from the maximal allowed baseline, determines negative scores (20–30 = −1; 30–40 = −2; 40–50 = −3; 50–60 = −4; >60 = −5 for men; 10 less for women and for all non-wine alcoholics: 10–20 = −1; 20–30 = −2; 30–40 = −3; 40–50 = −4; >50 = −5). Overall Adherence to Mediterranean Diet Score (AMDS) has a range of 0–55 and currently we consider adequate a score with a cut-off above 30/55.