Abstract
Objective: To identify gene expression changes and the role of activating transcription factor 3 (ATF3) in hemin toxicity in renal tubular epithelial cells, then elucidate molecular mechanisms of hemin toxicity on renal tubular epithelial cells.
Methods: An oligo array comprising 35,035 genes was used to compare differential gene expression in hemin-treated and non-treated HK-2 cells (human renal proximal tubular epithelial cells), and the role of ATF3 in hemin toxicity was assessed using siRNA technique.
Results: A total of 128 mRNAs were at least twofold up-regulated and 101 mRNAs were at least twofold down-regulated after hemin treatment. Expression levels of ATF3, heat shock protein 70, c-fos, and c-jun were remarkably increased. Hemin also suppressed nuclear factor-kappa B inhibitor α, β-2 adrenergic receptor, and interleukin-6 mRNA amounts more than twofold. We further demonstrated the protective role of ATF3 in hemin cytotoxicity.
Conclusions: The data suggest that hemin caused multiple changes of gene expression in HK-2 cells, and ATF3 protects against hemin cytotoxicity.
INTRODUCTION
Biological systems rely on heme proteins to carry out a number of basic functions essential for their survival.1 Heme proteins have a wide range of biological functions, including oxygen transport and storage (hemoglobin and myoglobin), electron transport (cytochromes), and catalysis (catalases and peroxidases).2 The versatility in the function of the heme group arises in particular from the different possibilities of axial ligation and interactions with the protein surrounding. Hemes, or iron–porphyrin complexes, are the versatile and ubiquitous active centers of these proteins.3 Heme is an iron-containing, reactive, lipophilic, tetrapyrrole ring that possesses such properties as the binding of oxygen and the redox cycling of its iron atom. In disease states, however, heme-containing proteins may be destabilized such that the linkage between the heme and the protein moieties may be weakened and ultimately sundered, and the cells and their organelles are thereby exposed to increased amounts of heme.4–9 The released heme exerts various noxious actions and injury occurs at multiple subcellular loci, which is underscored by the diversity and ubiquity of heme-containing proteins: hemoglobin, myoglobin, mitochondrial cytochromes, microsomal cytochromes, nitric oxide synthase, guanylate cyclase, prostaglandin synthase, catalase, glutathione peroxidase, NADPH oxidase, all contain the heme prosthetic group.10–13
Heme-mediated toxicity is particularly relevant to the kidney. This organ may be injured by increased amounts of heme from heme proteins present within the kidney, as occurs after ischemic and nephrotoxic insults.14,15 The kidney can also be damaged by large amounts of heme derived from heme proteins that originate elsewhere (such as myoglobin and hemoglobin) and are avidly taken up by the kidney, as occurs in such conditions as rhabdomyolysis and hemolysis.16,17 Spontaneous oxidation destabilizes hemoglobin or rhabdomyolysis, which results in the release of the heme moiety. The toxic effect of hemin on renal tubular epithelial cell is well established, and experimental evidence indicated that hemin intercalated into lipid membranes of the cells and greatly disturbed the bilayer structure of the cells, thus leading to cellular damage in erythrocytes and endothelial cells.16–20 Several studies showed hemin-induced oxidative stress that led to DNA damage, direct damage to proteins, glutathione (GSH) depletion, increases in intracellular free iron and copper, and increased lipoperoxidation. Increases in free iron leads to peroxidation and destruction of membranes and therefore releasing iron into the surrounding tissues, causing injury to nearby tissues and cells.21–25 Additionally, hemin has recently been recognized as a pro-inflammatory molecule, with particular attention to its ability to activate neutrophil responses. Exposure of endothelial cells to hemin stimulates the expression of intracellular adhesion molecules (ICAM-1), vascular cell adhesion molecule1 (VCAM-1), and endothelial leukocyte adhesion molecules (E-selectin).23–26 However, the exact mechanism or mechanisms of hemin toxicity and the predominant pathway of cell death remain to be defined.
To our knowledge, no unbiased search has been undertaken to assess overall gene expression alterations induced by hemin in renal tubular epithelial cells. Our research aimed to explore new mediators and targets of hemin action, and a gene expression profiling study was performed to identify novel mediators of hemin. Of the expressions of mRNAs altered in their expression, we were particularly intrigued by the induction of activating transcription factor 3 (ATF3). ATF3 encodes a member of the ATF/cAMP response element (CRE)–binding protein family of transcription factors that share the basic region-leucine zipper DNA-binding domain and bind to the same consensus sequence TGACGTCAC in vitro.24 ATF3 is a highly versatile stress sensor for a wide range of conditions including hypoxia, hyponutrition, oxidative stresses, ER stresses, various genotoxic stresses, and inflammatory reactions.27,28 Our work would help to clarify the molecular mechanisms of hemin-induced toxicity in renal tubular epithelial cells.
MATERIALS AND METHODS
Cell Culture and Treatment
The human kidney proximal tubular epithelial cell line (HK-2, Cell Culture Center of Chinese Academy of Medical Sciences, China) was cultured in DMEM (glucose 1.0 mmol/L) supplemented with 2 mM l-glutamine (Neuron Biotech, Beijing, China), 10% FBS, 15 mM HEPES, 50 U/mL penicillin, and 50 μg/mL streptomycin in a humidified 5% CO2 incubator at 37˚C. Cells were serially passaged every 4–5 days with 0.2% trypsin-EDTA. To assess the effects of hemin (Huayi Biotech, China) on HK-2 cells, hemin was dissolved in 0.1N NaOH, neutralized with 0.1N HCl to pH 8, then appropriate hemin solutions was added to the culture medium.
MTT Assay
The methyl thiazol tetrazolium (MTT) assay was used to determine cell viability. This assay relies on production of formazan by the action of mitochondrial enzymes on MTT by living cells and correlates well with other measures of cell number. Cells were exposed to the desired concentration of hemin for indicated time and then incubated with 0.5 mg/mL MTT (Amresco, solon, OH, USA) for 3 h. The formazan formed was solubilized in DMSO and quantitated by measuring the absorbance at 570 nm with ELISA reader (Bio-Rad, Hercules, CA, USA).
Human Genome Oligo Array
Human genome oligo array (35K) (Capital-Bio, Beijing, China) was used in this study. It comprises 35,035 mer oligo probes, each representing one transcript of the human genome. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol; RNA was purified using the NucleoSpin RNA clean-up kit (MachereyNagel, Düren, Germany). The concentration and quality of total RNA were measured by spectrophotometric absorbance (260 nm and 280 nm). RNA samples were reverse transcribed into single-strand cDNA, synthesized into double-strand cDNA and transcribed into cRNA using 17Ribo MAX Express Large Scale RNA Production System (Promega, Madison, WI, USA). After reverse transcription with random primers, cRNA products were marked with Klenow enzyme.
Samples were hybridized using a hybridization solution [25% formamide, 3× standard saline citrate (SSC), 0.2% sodium dodecyl sulfate (SDS), 5× Denhart’s] at 42˚C overnight in a humid environment. Subsequently, slides were washed on a horizontal shaker at 42˚C for 4 min, with washing solution (2× SCC). Arrays were scanned using Capital-Bio’s confocal scanner (LuxScan 10K-A, Beijing, China). An intensity-dependent loess program in the R language package was used to normalize the two channel ratio values. Statistical data and differential analysis files were generated (SAM software 3.0, USA).
Quantitative Polymerase Chain Reaction
Total RNA was isolated from cell culture using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was carried out using First Strand cDNA Synthesis Kit (Sangon, Shanghai, China) with 0.5 μg Oligo (dT)6, 50 mM Tris–HCl (pH 8.3 at 25˚C), 75 mM KCl, 75 mM KCl, 3 mM MgCl2, 50 mM DTT, 10 mM each free dNTP, and 100 U of MMLV reverse transcriptase according to the manufacturer’s instruction. Quantitative polymerase chain reaction (QPCR) was done with Applied Biosystems 7300 Real-Time PCR system (Foster City, CA, USA) and SYBR Green kit (Kangcheng Biotech, Beijing, China) containing 50 mM KCl, 20 mM Tris–HCl (pH 8.4), 0.2 mM each free dNTP, hot start enzyme Taq DNA polymerase (25 U/mL), 3 mM MgCl2, SYBR Green 1, and 10 nM fluorescein as passive reference. GTP-binding protein (GEM), regulator of G-protein signaling 2 (RGS2), immediate-early response gene 5 (IER5), oxidative-stress growth inhibitor-1 (OSGIN1), and interleukin (IL)-6 were selected for validation. The primers were as follows: GEM: 5′-TCAATCACAGACCGAGCGAG-3′, 5′-CCTCAAACAGCTCCTTCACGT-3′; RGS2: 5′-ATTGGAAGACCCGTTTGAGC-3′, 5′-CAGCAAGACCATAT-3′; IER5: 5′-CTTCGGTTCCAGTTTCTCGG-3′, 5′-TGCTCCAGGGGTTCATGTCT-3′; OSGIN1: 5′-GGGAAACATGAAGTCGGTCC-3′, 5′-CCCTGTAGTAGTGGGCGATGT-3′; IL-6: 5′-ATGAACTCCTTCACAAG-3′, 5′-TGTCAATTCGTTCTGAAGAG-3′; Actin: 5′-CATGTACGTTGCTATCCAGGC-3′, 5′-CTCCTTAATGTCACGCACGAT-3′. Transcripts were amplified in duplicates with specific sense and antisense primers. The program for thermal cycle was 10 min at 95˚C followed by 40 cycles of 15 s at 95˚C, 30 s at 55˚C, and 33 s at 72˚C. The melting points of PCR product were determined by incubating at 65˚C for 1 min followed by a 1˚C per min rise over 30 min. The cycle at which the baseline level is exceeded is defined as the threshold cycle (CT). The value stands for 2-ΔΔCT. The reverse transcription reaction was performed on 2 μg total RNA according to the manufacturer’ s instruction. Transcripts were detected with the SYBR Green kit (Kangcheng Biotech, Beijing, China).
Immunocytochemistry
Cells plated on glass coverslips were fixed in ethanol–acetone mixture (1:1) for 15 min at 4˚C, rinsed three times with PBS, permeabilized with 0.1% trypsin for 1 min at room temperature, and rinsed with PBS. Samples were blocked in blocking buffer (10% goat serum in PBS) for 10 min, then incubated with primary antibody (Santa Cruz, CA, USA) at 4˚C overnight. After being rinsed with PBS, the samples were treated with a HRP-conjugated secondary antibody for 1 h at room temperature. After removing the secondary antibody, the samples were rinsed with PBS, then DAB staining was performed.
RT-PCR
Cells were harvested, and total RNA was extracted and used for reverse transcription. Aliquots of 1.5 µL cDNA were subsequently amplified in a total volume of 20 µL using the PCR kit (TIANGEN, Beijing, China) following conditions recommended by the manufacturer. The sense and antisense primers for ATF3 were 5′-GTTTGAGGA TTTT GCTAACCTGAC-3′ and 5′-AGCTGCAATCTTATTT CTTTCT CGT-3′ (211 bp), respectively. The sense and antisense primers for the GADPH gene used as an internal control were 5′-AGGGCTGCTTTTAACTCTGGT-3′ and 5′-CCCCACTTGATTTTGGAGGGA-3′ (206 bp), respectively. The cycling conditions were 94˚C for 4 min, followed by 30 cycles at 94˚C for 30 s, at 60˚C for 30 s, and at 72˚C for 1 min and a final extension at 72˚C for 10 min. PCR products were separated on the 1.5% agarose gel stained with ethidium bromide and viewed under ultraviolet light.
siRNA Design and Transfection
Control siRNA and siRNA for ATF3 were designed as follows: control siRNA: 5′-UUCUCCGAACGUTT-3′, 5′-ACGUGACACGUUCGGAGAATT-3′; ATF3-siRNA: 5′-GGUUUGCCAGAACAATT-3′, 5′-UUGUUCUGGAUGGCAAACCTT-3′. One picomole of the siRNA dissolved in 500 μL of culture medium was transfected into HK2 cells using 20 μL Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
Caspase-3 Activity Assay
Cultured cells were lysed in radio-immunoprecipitation assay buffer, all samples were spun (12,000 rpm) for 15 min at 4˚C, and the protein concentration in each lysate was determined by Bradford Protein Assay Kit (Beyotime Biotech, Shanghai, China). The caspase-3 activities of the protein samples were assessed with caspase-3 activity assay kit (Beyotime). The reaction system including 20 μL sample and 10 μL acetyl-aspirate-glutamate-valine-aspirate p-nitroaniline (Ac-DEVD-Pna) were incubated at 4˚C for 1.5 h. Absorbance at 405 nm was measured with ELISA reader (Bio-Rad).
Statistics
All statistical analyses were performed using SPSS version 15.0. The results were presented as the mean ± SD for the hemin-treated and non-treated groups. One way analysis of variance or Student’s t-test was performed to verify significant differences between the groups in microarray and RT-PCR results. For all tests, statistical significance was established as a p-value of <0.05.
RESULTS
Toxic Effect of Hemin on HK-2 Cells
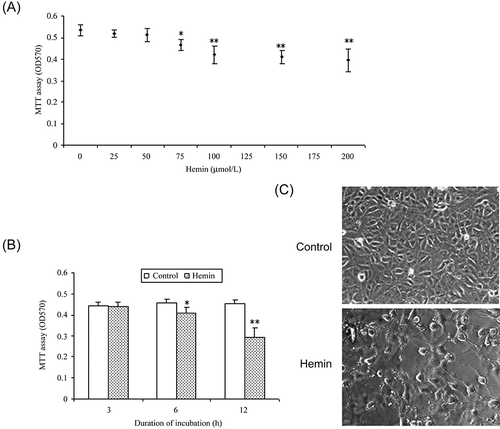
At the doses of 50 μmol/L, hemin treatment for 6 h had little effect on cell viability; at the doses of 75–200 μmol/L, hemin treatment for 6 h led to a significant reduction of viable cells in a dose-dependent manner (A), To analyze the time-course effects of hemin, we used 75 μmol/L of hemin to avoid severe cell loss; the results showed that hemin exerted minor effect at 3 h, while at 6 and 12 h, hemin induced a significant decrease in cell viability compared with control in a time-dependent manner (B). After 75 μmol/L hemin treatment for 6 h, cell shrinkage occurred (C)
Figure 1. Hemin-induced cell death in HK-2 cells. (A) Cytotoxicity effects of different concentrations of hemin (6 h treatment) on HK-2 cells. (B) Time course of cytotoxic effect of 75 μmol/L hemin on HK-2 cells. Each value is expressed as mean ± SD. *p < 0.05, **p < 0.01 compared to vehicle-treated group. (C) Morphological changes of 75 μmol/L hemin treatment for 6 h in HK-2 cells observed with phase contrast microscope (bar = 25 μm).
Expression Profiling Identification of Hemin Targets Up-Regulated in Their Expression
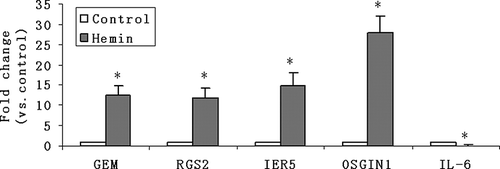
To identify changes in gene expression levels, HK-2 cells were treated with and without 75 μmol/L hemin for 6 h; a total of 128 mRNAs were at least twofold up-regulated in response to hemin. Of the genes up-regulated by hemin, some were of potential interest for their recognized role in apoptosis, cell proliferation, inflammatory response, oxidative stress, G-protein-mediated signaling pathway, mitogen-activated protein kinase (MAPK) signaling pathway, and so on. Some of these data are illustrated in . It is noteworthy that heat shock protein 70-6 (HSP70-6) mRNA increased by 34.5-fold (the strongest up-regulation); moreover, mRNAs for other HSP family such as HSP70-1 and HSP60 were also induced by hemin.
Table 1. Genes up-regulated in expression by hemin.
Evidence showed that the AP-1 binding motif plays a critical role in mediating hemin action,29 and the heme-responsive element of the mouse heme-oxygenase-1 gene is an extended AP-1 binding site. The transcripts of immediate-early genes c-fos and c-jun (bZIP proteins) were transiently up-regulated; the protein products of these two genes constitute the AP-1 transcription factor complex. Other immediate-early response genes such as immediate-early response gene 5 were also shown to be up-regulated, and the oxidative stress-related genes such as oxidative stress-induced growth inhibitor 1 were also strongly induced.
Evidence previously demonstrated that hemin could stimulate cAMP production in human peripheral blood mononuclear cells, induce activation of extracellular regulation kinases (ERK) in hemin-responsive K-562 cells, and activate phosphate-dylinositol 3-kinase signaling pathway.30–32 However, to date, a systematic investigation of the signaling pathways regulated by hemin has not been reported. Our results found that transcripts of RGS10, RGS17, and small G-proteins (Gem and Rit) remarkably increased, suggesting that G-protein and small G-protein signaling pathways might be involved in hemin action.
In addition, we have an interesting finding that testin gene expression was elevated after hemin treatment. Testin was reported to be localized in various tissues including kidney, and testin expression in human renal tubular epithelial cell was induced by hemin.33 It was reported that red meat consumption affects sex hormone level; we found that hemin, as prosthetic group of myoglobin in red meat, increased testin expression, and this might provide an insight into the mechanism of the impact of red meat on sex hormone.
Expression Profiling Identification of Hemin Targets Attenuated in Their Expression
A total of 101 mRNAs were at least twofold down-regulated after hemin treatment. Of all the mRNAs down-regulated by hemin (), β-2 adrenergic receptor was the most dramatically attenuated (8-fold).
Table 2. Genes down-regulated in expression by hemin.
We also observed reductions in the amount of mRNA encoding signal transducer and activator of transcription (STAT1) and transforming growth factor-β2, and their roles in hemin toxicity have not been reported to date. Hemin also suppressed nuclear factor-kappa B (NF-κB) inhibitor α (IκBα) and IL-6 mRNA amounts more than twofold. It was reported that hemin could increase NFκB expression and stimulate DNA-binding activity of NFκB34; this finding suggests that the repression of IκB might be involved in the hemin-induced NFκB activation.
The gene expression profiling studies also revealed reductions in the mRNAs encoding G1/S-specific cyclin-D2 and cyclin-A; thus, together with the elevation of p21 expression (), we postulated that hemin might affect cell proliferation and apoptosis partly through regulation of transcription of cyclin family.
We also observed that hemin decreased amount of mRNA encoding the transferrin receptor protein 1 (TfR1), the major iron uptake protein. We postulated that the hemin-induced TfR1 repression might be involved in protecting cells from an iron overload because of hemin dissociation.
The up-regulated genes (such as GEM, RGS2, IER5, and OSGIN1) and the down-regulated genes (represented by IL-6) after hemin treatment were confirmed with QPCR analysis.
Validation of ATF3 Up-Regulated Expression
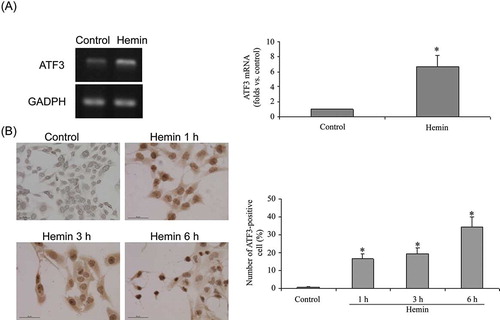
The expression profiling data revealed that ATF3 was elevated by 9.44-fold. ATF3 was activated by a variety of extracellular stresses,35,36 and was implicated to be hub of the cellular adaptive–response network in response to different stimuli. Thus, experiments were performed to validate the expression profiling data. Hemin at 75 μmol/L for 6 h elevated ATF3 gene expression (A). Hemin increased the number of ATF3-positive cells in a time-dependent manner at the concentration of 75 μmol/L; ATF3-positive cells were very few in non-treated cells. However, after hemin treatment, the number of ATF3-staining cells remarkably increased within 1–6 h, and the increase in ATF3 mRNA was evident at 6 h of hemin treatment (B). Western blot analysis will be performed in our future study.
Figure 3. Expression of ATF3 in HK-2 cells. (A) ATF3 mRNA expression was determined with RT-PCR. Each value is expressed as mean ± SD. *p < 0.001 compared to vehicle-treated group. (B) The localization of ATF3 in hemin-treated and non-treated cells was determined by immuno- cytochemistry with ATF3 antibody (bar = 50 μm). Each value is expressed as mean ± SD. *p < 0.001 compared to vehicle-treated group.
Effect of ATF3-siRNA on Hemin Toxicity
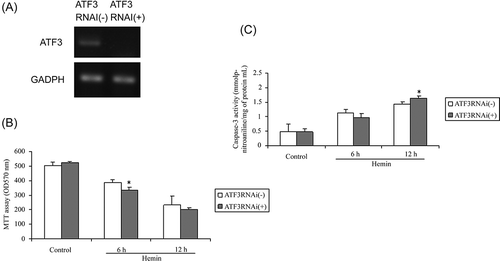
To address the role of ATF3 in hemin toxicity, siRNA was transfected to knockdown ATF3 in HK-2 cells, then treated with and without hemin. As shown in A, ATF3-siRNA remarkably inhibited ATF3 gene expression. ATF3-siRNA had little effect on cell viability in the absence of hemin, while significantly reduced the cell viability in the presence of hemin (B). To further demonstrate the protective function of ATF3, caspase-3 (an apoptosis marker) activity was measured. ATF3-siRNA alone had minimal effect on caspase-3 activity. However, after hemin treatment, stronger caspase-3 activity was observed in ATF3-siRNA transfected cells than in cells transfected with scramble siRNA (C), suggesting that ATF3 protected HK-2 cells against hemin toxicity.
Figure 4. Effects of ATF3-siRNA on hemin toxicity in HK-2 cells. (A) ATF3-siRNA reduced ATF3 gene expression. (B) ATF3-siRNA reduced cell viability after 75 μmol/L hemin treatment for 6 h. (C) ATF3-siRNA augmented caspase-3 activity after 75 μmol/L hemin treatment for 12 h. Each value is expressed as mean ± SD. *p < 0.05 compared to control siRNA-treated group.
DISCUSSION
Heme is the functional group of various proteins, including hemoglobin, myoglobin, cytochromes, and nitric oxide synthases.37,38 Under some pathological conditions or oxidative stress, heme may be released and exert various noxious actions. Hemin is quite hydrophobic, readily intercalates into lipid membranes of cells, and greatly disturbs the bilayer structure of the cells, thus leading to cellular damage in erythrocytes and endothelial cells.39–44 The toxic effect of hemin on renal tubular cell is well established, and several molecular targets of hemin have previously been identified. Despite the recognition that heme accumulates in the injured kidney and may be damaging, the influence of hemin on the gene expression alterations remains unknown. A crucial step towards understanding molecular mechanisms underlying stress responses is the identification of target genes of each transcription factor. In this study, we used an mRNA expression profiling approach to identify several potential new targets of hemin.
The broad-spectrum distribution of the differentially expressed genes indicates that multiple pathways may be involved in HK-2 cells resulting after hemin treatment. The c-fos and c-jun mRNA levels were elevated after being treated with hemin, and increased levels of 11 mRNAs (HSP family) were also noted. HSPs were reported to protect against experimental renal injury, and accumulating evidence showed that hemin was an inducer of HSP.45 Specific mRNAs encoding HSP were identified in this study, which might be helpful in giving more information. Previous study showed that hemin increased NF-κB expression and its DNA-binding activity, and the down-regulation of IκB was observed after hemin treatment in this study; our findings might shed new light on how hemin regulate NF-κB.
Of the up-regulated mRNAs, we took much interest in ATF3, which is a member of the ATF/CREB family and is known to be activated under a variety of extracellular stresses. We provide evidence herein that ATF3 is involved in cellular response to hemin by promoting cell survival.
Experimental evidence showed that ATF3 mRNA levels are relatively low in most cells, while elevated in response to a variety of stimuli including stress and growth factors. In this study, we first reported hemin-induced ATF3 expression in renal epithelial HK-2 cell. It was reported that ATF2 and c-jun could significantly stimulate ATF3 promoter activity; moreover, ERK and JNK pathway are involved in ATF3 induction.46 Considering increased c-jun expression was observed in this experiment, we speculated that hemin treatment induced ATF3 probably via c-jun activation.
ATF3 appears to play distinct roles in different cell types in response to different stimuli. For example, expression of ATF3 was beneficial by helping pancreatic β-cells to cope with higher metabolic demand and attenuating allergic pulmonary inflammation,47,48 ATF3 cDNA synergized with curcumin in inducing apoptosis.49 Here our results showed ATF3 silencing aggravated hemin-induced injury in HK-2 cells.
Although the present study identified that ATF3 protected against hemin-induced injury in renal epithelial cells, further work will be needed to clarify its mechanisms of action. The heterodimer of ATF3/c-jun can induce HSP27, which activates the Akt pathway and inhibits MEKK1-JNK, thereby suppressing apoptosis in renal cells.50 Based on the results of ATF3 silencing and microarray data, we postulated that the effect of ATF3 might be partly mediated by HSPs, but we cannot exclude the possibility that other genes might also be involved in response to hemin.
Taken together, by using an unbiased approach, we revealed the overall gene expression changes in response to hemin, which might provide important insights into the study on hemin toxicity. Importantly, we found differential ATF3 expression played a protective role in hemin-induced injury in renal epithelial cells and may help to point toward new avenues for the therapeutic treatment. Our next work will further demonstrate the mechanisms underlying the protective effects of ATF3.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Paoli M, Marles-Wright J, Smith A. Structure-function relationships in heme-proteins. DNA Cell Biol. 2002;21(4):271–280.
- Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996;6:129–168.
- Nienhaus K, Zosel F, Nienhaus GU. Ligand binding to heme proteins: a comparison of cytochrome C variants with globins. J Phys Chem B. 2012;116(40):12180–12188.
- Immenschuh S, Baumgart-Vogt E, Mueller S. Heme oxygenase-1 and iron in liver inflammation: a complex alliance. Curr Drug Targets. 2010;11(12):1541–1550.
- Soudi M, Zamocky M, Jakopitsch C, Furtmüller PG, Obinger C. Molecular evolution, structure, and function of peroxidasins. Chem Biodivers. 2012;9(9):1776–1793.
- Zager RA, Johnson AC, Becker K. Renal cortical hemopexin accumulation in response to acute kidney injury. Am J Physiol Renal Physiol. 2012;303(10):F1460–F1472.
- Zarjou A, Agarwal A. Heme oxygenase-1 as a target for TGF-β in kidney disease. Semin Nephrol. 2012;32(3):277–286.
- Zhou J, Ouyang X, Schoeb TR, et al. Kidney injury accelerates cystogenesis via pathways modulated by heme oxygenase and complement. J Am Soc Nephrol. 2012;23(7):1161–1171.
- Elmarakby AA, Faulkner J, Baban B, Saleh MA, Sullivan JC. Induction of hemeoxygenase-1 reduces glomerular injury and apoptosis in diabetic spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2012;302(7):F791–F800.
- Hill-Kapturczak N, Chang SH, Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol. 2002;21:307–321.
- Kanakiriya SK, Nath KA. Heme oxygenase and acute renal injury. In: Molitoris B and Finn WF, eds. Acute Renal Failure: A Companion to Brenner and Rector’s the Kidney. 1st ed., ch. 5. Philadelphia, PA: Saunders; 200178–88.
- Muller-Eberhard U, Fraig M. Bioactivity of heme and its containment. Am J Hematol. 1993;42:59–62.
- Stocker R. Induction of hem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 1990;9:101–112.
- Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49:314–326.
- Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol. 2000;11:965–973.
- Jeney V, Balla J, Yachie A, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887.
- Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. Hemin induces an iron-dependent, oxidative injury to human neuron-like cells. J Neurosci Res. 2003;73:113–121.
- Balla J, Balla G, Jeney V, Kakuk G, Jacob HS, Vercellotti GM. Ferriporphyrins and endothelium: a 2-edged sword-promotion of oxidation and induction of cytoprotectants. Blood. 2000;95(4):3442–3450.
- Wagener FA, Eggert A, Boerman OC, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–1811.
- Jeney V, Balla J, Yachie A, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887.
- Dennery PA, Sridhar KJ, Lee CS, et al. Heme oxygenase-mediated resistance to oxygen toxicity in hamster fibroblasts. J Biol Chem. 1997;272:14937–14942.
- Hebbel RP, Eaton JW. Pathobiology of heme interaction with the erythrocyte membrane. Semin Hematol. 1989;26:136–149.
- Iwata M, Zager RA. Myoglobin inhibits proliferation of cultured human proximal tubular (HK-2) cells. Kidney Int. 1996;50:796–804.
- Lavrovsky Y, Schwartzman ML, Levere RD, Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase1 gene. Proc Natl Acad Sci USA. 1994;91:5987–5991.
- Laird MD, Wakade C, Alleyne CH, Dhandapani KM. Hemin-induced necroptosis involves glutathione depletion in mouse astrocytes. Free Radic Biol Med. 2008;45:1103–1114.
- Chowa JM, Huang GC, Lin HY, Shen SC, Yang LY, Chen YC. Cytotoxic effects of metal protoporphyrins in glioblastoma cells: roles of albumin, reactive oxygen species, and heme oxygenase-1. Toxicol Lett. 2008;177:97–107.
- Yin X, Wolford CC, Chang YS, et al. ATF3, an adaptive-response gene, enhances TGF signaling and cancer-initiating cell features in breast cancer cells. J Cell Sci. 2010;123(20):3558–3565.
- Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: is modulation of inflammation a unifying component? Gene Expr. 2010;15(1): 1–11.
- Hasan RN, Schafer AI. Hemin up-regulates Erg-1 expression in vascular smooth muscle cell via reactive oxygen species, ERK-1/2. Elk-1 and NF-KB. Circ Res. 2008;102:42–50.
- Lander HM, Levine DM, Novogrodsky A. Hemin stimulation of cAMP production in human lymphocytes. FEBS Lett. 1992;303(2–3):242–246.
- Woessmann W, Mivechi NF. Role of ERK activation in growth and erythroid differentiation of K562 cells. Exp Cell Res. 2001;264(7):193–200.
- Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–184.
- Grima J, Zhu LJ, Zong SD, Catterall JF, Bardin CW, Cheng CY. Rat testin is a newly identified component of the junctional complexes in various tissues who- se mRNA is predominantly expressed in the testis and ovary. Biol Reprod. 1995;52:340–355.
- Shih HJ, Yang Y, Huang CJ, Chow YC. Hemin-induced HO-1 over expression after testicular torsion-detorsion involeves NRF2, NF-κB, ERK. Eur Urol Suppl. 2008;7(3):91.
- Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11.
- Liang G, Wolfgang CD, Chen BP, Chen TH, Hai T. ATF3 gene. Genomic organization, promoter, and regulation. J Biol Chem. 1996;271:1695–1701.
- Beri R, Chandra R. Chemistry and biology of heme: effect of metal salts, organometals, and metalloporphyrins on heme synthesis and catabolism, with special reference to clinical implications and interactions with cytochrome P-450. Drug Metab Rev. 1993;25:149–152.
- Fitch CD, Chevli R, Kanjanangglupan P, Dutta P, Chevli K, Chou AC. Intracellular ferriprotoporphyrin IX is a potent lytic agent. Blood. 1983;62:1165–1168.
- Shaklai N, Shviro E, Rabizadeh E, Kirschner-Zibler I. Accumulation and drainage of hemin in the red cell membrane. Biochim Biophys Acta. 1985;821:355–366.
- Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64:648–655.
- Liu SC, Zhai S, Lawler J, Palek J. Hemin-induced dissociation of erythrocyte membrane skeletal proteins. J Biol Chem. 1985;260:12234–12239.
- Harvey JW, Beutler E. Binding of heme by glutathione Stransferase: a possible role of the erythrocyte enzyme. Blood. 1982;60: 1227–1230.
- Zaidi A, Marden MC, Poyart C, Leclerc L. Protection by lazaroids of the erythrocyte (Ca2+, Mg2+)-ATPase against iron-induced inhibition. Eur J Pharmacol. 1995;290:133–139.
- Sullivan SG, Baysal E, Stern A. Inhibition of hemin-induced hemolysis by desferrioxamine: binding of hemin to red cell membranes and the effects of alteration of membrane sulfhydryl groups. Biochem Biophys Acta. 1992;1104:38–44.
- Zhang PL, Lun MY, Schworer CM, et al. Heat shock protein expression is highly sensitive to ischemia-reperfusion injury in rat kidneys. Ann Clin Lab Sci. 2008;38(1):57–64.
- Cai Y, Zhang C, Nawa T, et al. Homocysteine responsive ATF3 gene expression in human vascular endothelial cells: activation of c-Jun NH (2) terminal kinase and promoter response element. Blood. 2000;96(6):2140–2148.
- Zmuda EJ, Qi L, Zhu MX, Mirmira RG, Montminy MR, Hai T. The roles of ATF3, an adaptive-response gene, in high-fat-diet-induced diabetes and pancreatic β-Cell dysfunction. Mol Endocrinol. 2010;24:1423–1433.
- Gilchrist M, Henderson WR Jr, Clark AE, et al. Activating transcription factor 3 is a negative regulator of allergic pulmonary inflammation. J Exp Med. 2008;205:2349–2357.
- Yan CH, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther. 2005;4:233–241.
- Nakagomi S, Suzuki Y, Namikawa K, Kiryu-Seo S, Kiyama H. Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J Neurosci. 2003;23:5187–5196.